A Novel Solution for Vitamin K1 and K2 Analysis in Human Plasma by LC-MS/MS
Abstract
Vitamin K1 and K2 analysis is typically complex and time-consuming because these lipophilic vitamins occur at low levels and are subject to matrix interference, particularly from phospholipids. The new method established here provides fast, accurate, and precise vitamin K analysis in plasma using a simple phospholipid removal procedure and a sensitive, 4-minute LC-MS/MS analysis on a Raptor Biphenyl column, making it suitable for both research and high-throughput testing.
Introduction
Vitamin K is a group of fat-soluble vitamins divided into vitamin K1 (one compound, phylloquinone) and K2 (a group of compounds, menaquinones). Among the K2 family, MK4 and MK7 are the most nutritionally recognized. While K1 plays an important role in controlling blood clotting, studies have shown that MK4 and MK7 have distinct biological function in the regulation of bone metabolism and vascular calcification. As interest in their biological action in extra-hepatic tissues is increasing, an accurate and simple measurement of vitamin K status remains a critical issue for both clinical research and diagnostics. Vitamin K1 and K2 analysis is extremely challenging because they are the most lipophilic and least abundant of the fat-soluble vitamins. Common strategies are complex and time-consuming: typical methods use liquid-liquid extraction paired with silica-based SPE to separate polar and nonpolar lipids, followed by post-column derivatization and fluorescence detection. In this study, a simple and fast phospholipid removal sample preparation procedure was developed and used in combination with an LC-MS/MS analysis using a Raptor Biphenyl column. The method established here provides a novel solution for vitamin K measurement for both research and high-throughput clinical diagnostic labs.
Figure 1: Structures of Vitamin K1 and K2
Experimental
Calibration Standards and Quality Control Samples
SeraFlx BIOMATRIX (BioIVT) was fortified with the three vitamin K analytes to prepare calibration standards and QC samples. Seven-point calibration curves established a 0.10-10 ng/mL linear range. Three QC levels were prepared at 0.25, 0.75, and 8.00 ng/mL for all three vitamin K analytes. The fortified standards and QC samples were subjected to sample preparation procedure as described further on.
Human Plasma Analysis
To ensure method applicability across a range of patient populations that vary in endogenous vitamin K levels, multiple human plasma samples (BioIVT) were analyzed. These samples included pooled plasma (from 20 people), plasma from an individual taking vitamin K2 medication, and plasma from an individual of a Japanese descent because the typical Japanese diet is high in MK7-containing foods.
Sample Preparation
A 500 µL aliquot of sample was mixed with 5 µL of internal standard solution (K1-d7, MK4-d7, and MK7-d7 at 100 ng/mL in methanol) and 1.5 mL of acetonitrile followed by vortexing for 20 seconds at 3000 rpm. After centrifugation at 4300 rpm for 10 minutes, the supernatant was loaded onto a Biotage ISOLUTE PLD+ 96-well plate (50 mg) and vacuum was applied to collect the eluate. The eluate was then evaporated to dryness at 50 °C under a gentle stream of nitrogen. The dried extract was reconstituted with 100 μL of 15:85 water:methanol and 5 μL of sample was injected for analysis.
Instrument Conditions
LC-MS/MS analysis of vitamin K1 and K2 in human plasma was performed on a Waters Xevo TQ-S mass spectrometer paired with an ACQUITY UPLC system. Instrument conditions were as follows, and analyte transitions are provided in Table I.
| Analytical column: | Raptor Biphenyl 2.7 µm, 50 mm x 2.1 mm (cat.# 9309A52) | |
| Mobile phase A: | Water, 0.1% formic acid, 5 mM ammonium formate | |
| Mobile phase B: | Methanol, 0.1% formic acid | |
| Gradient: | Time (min) | %B |
| 0.00 | 90 | |
| 1.00 | 100 | |
| 3.00 | 100 | |
| 3.01 | 90 | |
| 4.00 | 90 | |
| Flow rate: | 0.4 mL/min | |
| Injection volume: | 5 µL | |
| Column temp.: | 40 °C | |
| Ion mode: | Positive ESI | |
| Analyte | Precursor Ion | Product Ion Quantifier | Product Ion Qualifier |
|---|---|---|---|
| MK4 | 445.5 | 187.2 | 81.1 |
| K1 | 451.5 | 187.2 | 128.2 |
| MK7 | 649.7 | 187.2 | 81.1 |
| MK4-d7 | 452.5 | 194.2 | - |
| K1-d7 | 458.5 | 194.2 | - |
| MK7-d7 | 656.7 | 194.2 | - |
Results and Discussion
Chromatographic Performance
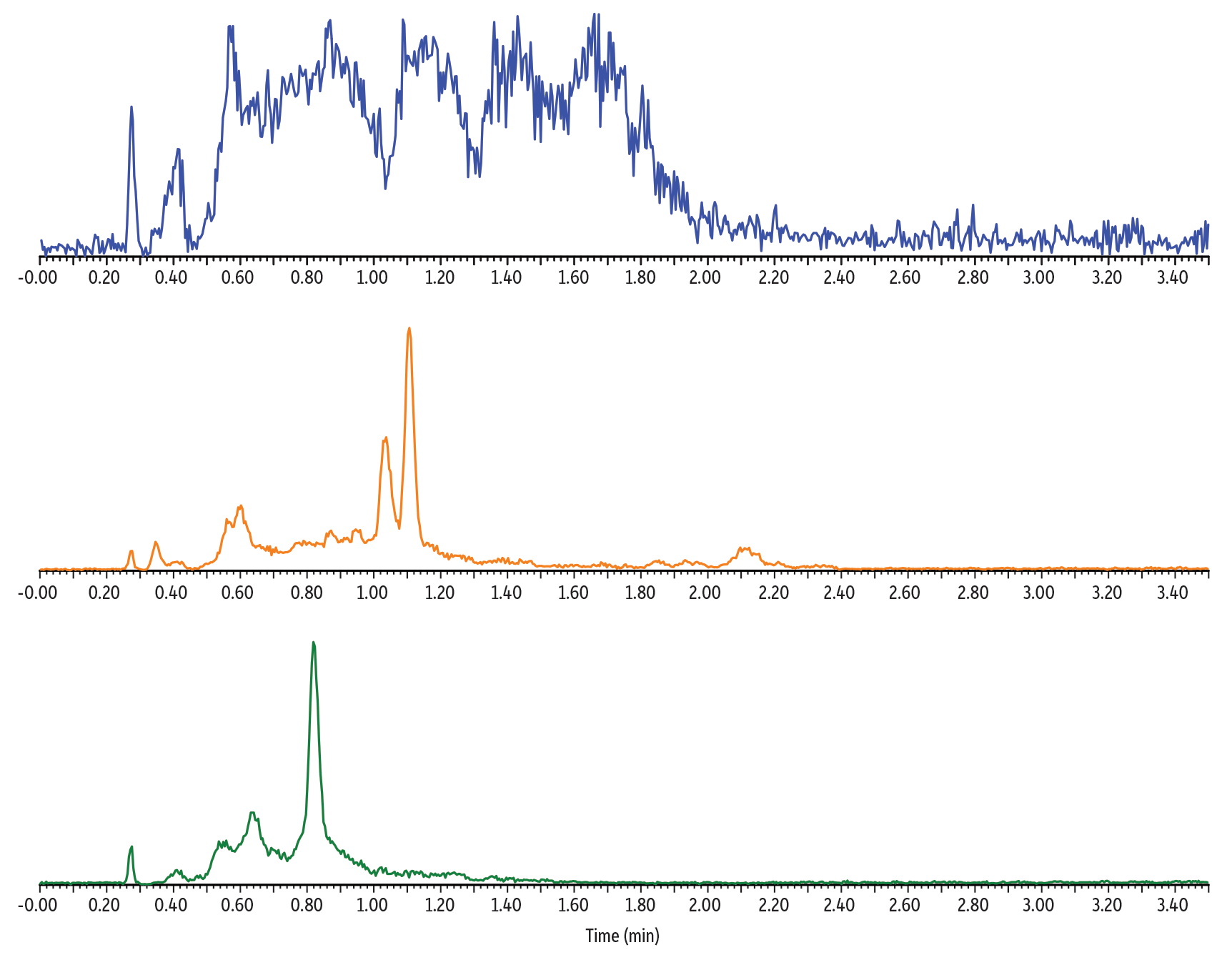
A fast, 4-minute chromatographic vitamin K1 and vitamin K2 analysis was achieved with injection of reconstituted solution obtained from a simple phospholipid removal procedure. No chromatographic interferences were observed from the analysis of blank plasma samples (Figure 2), indicating the technique provided sufficient removal of matrix components.
Figure 2: Analysis of Blank and Fortified SeraFlx BIOMATRIX
Blank
0.5 ng/mL Fortified
Linearity
Using 1/x weighted linear regression, all three analytes showed acceptable linearity with r2 values of 0.995 or greater, and deviations of <15% from the nominal concentrations. The established limit of quantitation was 0.10 ng/mL. Representative standard curves are shown in Figure 3.
Figure 3: Standard Curves
Accuracy and Precision
Precision and accuracy testing for vitamin K1 and K2 analysis according to the method established here was performed on three different days. The method was demonstrated to be accurate with recovery values falling within 10% of the nominal concentration for all QC levels. The %RSD was 0.207-7.83% and 3.39-5.75% for intraday and interday assessments, respectively, indicating acceptable method precision (Table II).
Table II: Accuracy and Precision of QC Samples for Vitamin K1 and K2 Analysis
| QC-1 (0.250 ng/mL) | QC-2 (0.750 ng/mL) | QC-3 (8.00 ng/mL) | |||||||
| Analyte | Avg. Conc. (ng/mL) | Avg. Accuracy (%) | Precision (%RSD) | Avg. Conc. (ng/mL) | Avg. Accuracy (%) | Precision (%RSD) | Avg. Conc. (ng/mL) | Avg. Accuracy (%) | Precision (%RSD) |
| K1 | 0.246 | 98.2 | 5.75 | 0.763 | 102 | 3.39 | 8.23 | 103 | 4.36 |
| MK4 | 0.249 | 100 | 4.55 | 0.729 | 97.2 | 3.83 | 8.11 | 101 | 5.17 |
| MK7 | 0.245 | 98.0 | 3.88 | 0.715 | 95.3 | 4.98 | 8.23 | 103 | 4.76 |
Human Plasma Analysis
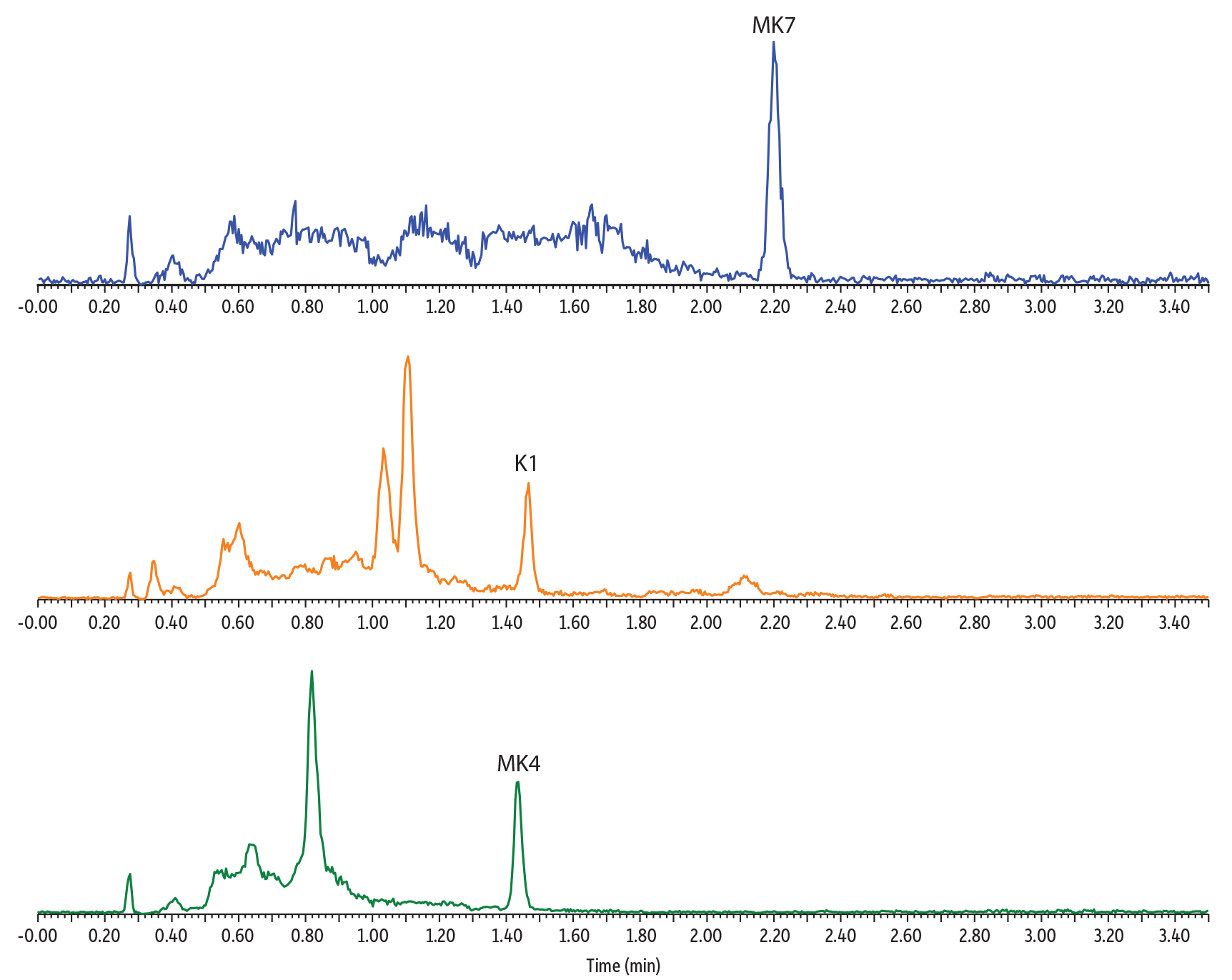
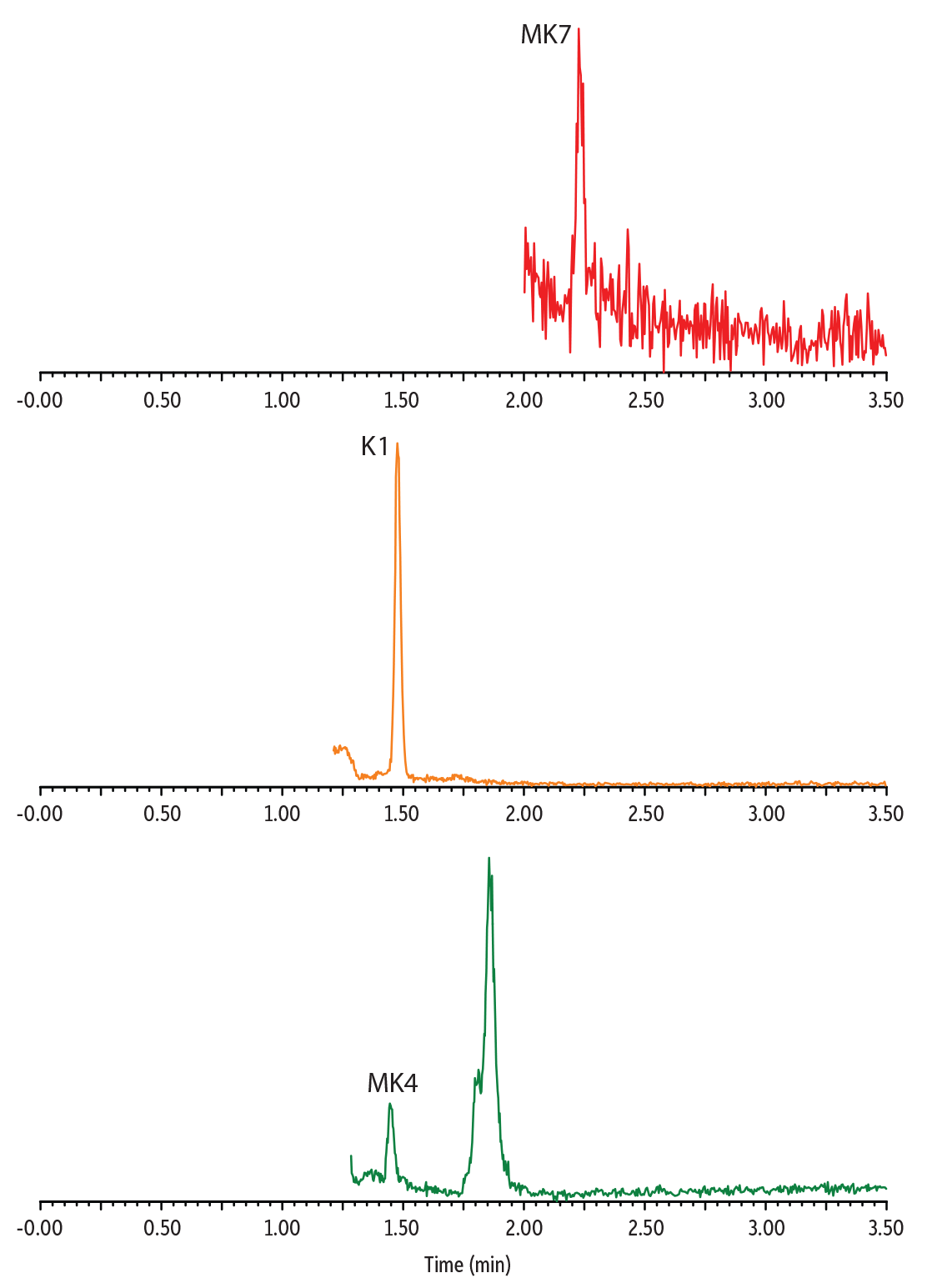
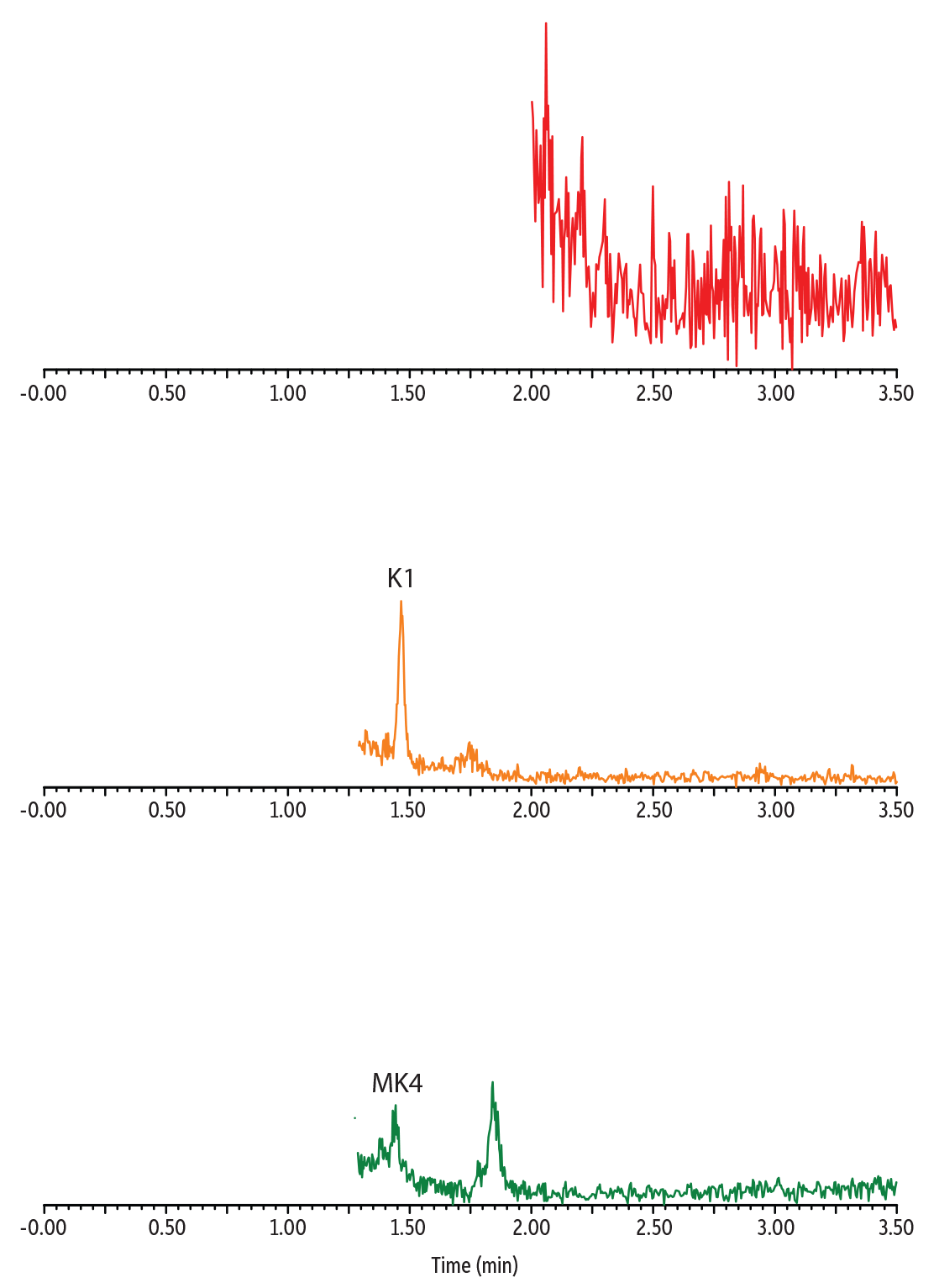
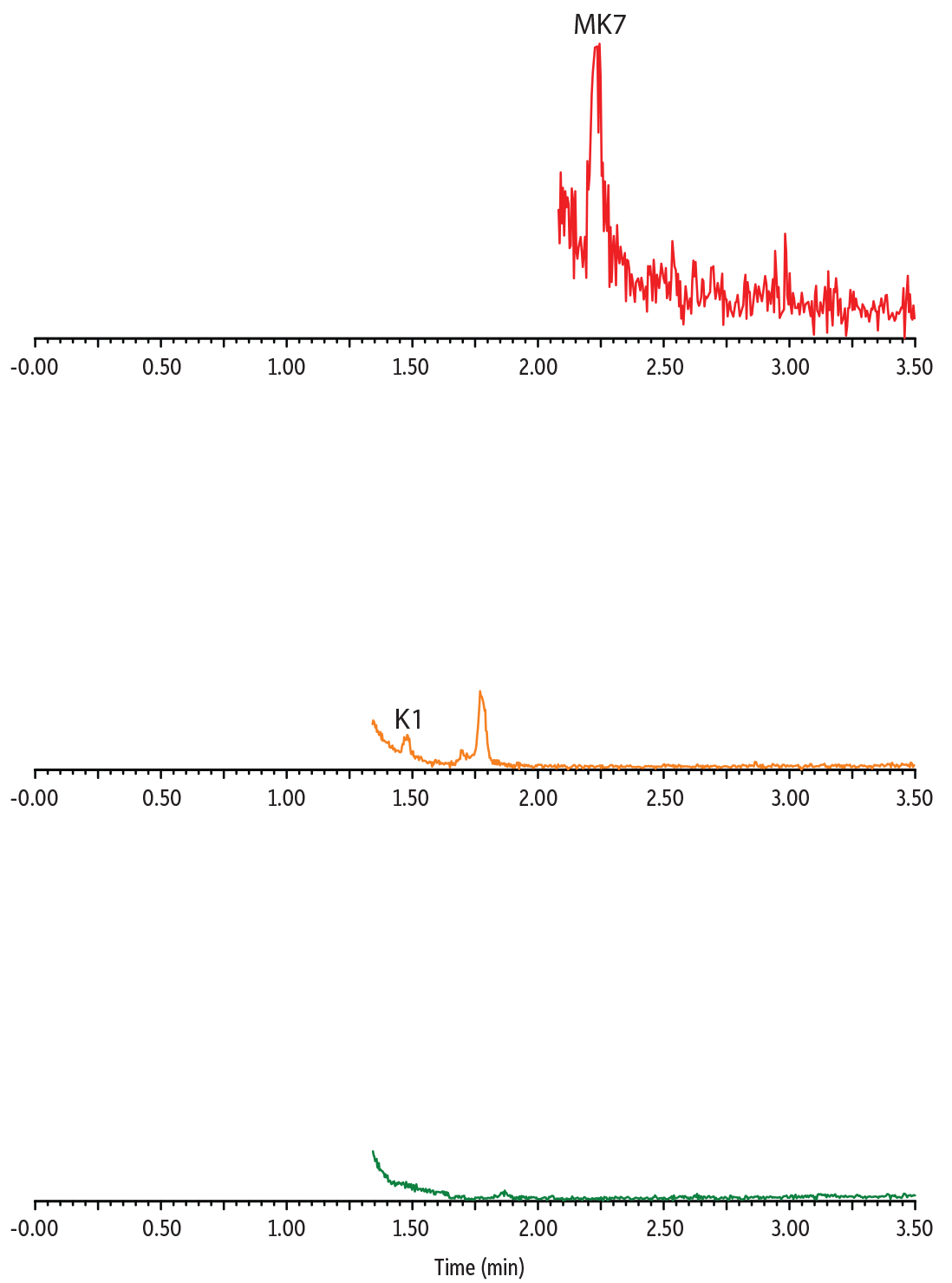
Distinct endogenous vitamin K profiles were revealed by analyzing vitamin K1 and vitamin K2 in multiple plasma samples (Figure 4). The analysis of pooled human plasma is a good indication of the average vitamin K level of a collected population. The result showed clear detection of K1 and MK4 in pooled plasma with no detectable MK7, reflecting that the collected population has very low consumption of the MK7-containing food. Conversely, a plasma sample from an individual of Japanese descent showed higher MK7 and no detectable MK4. The plasma sample from an individual taking vitamin K2 medication showed strong K1, MK4, and MK7 signals. Overall, these results demonstrated that our established method can measure the variation of vitamin K1 and K2 concentrations seen in different populations and is thus applicable for a broad range of clinical samples.
Figure 4: Analysis of Endogenous Vitamin K1 and K2 in Human Plasma
Plasma from Patient Taking Vitamin K2 Medication
Pooled Human Plasma Sample
Plasma from Individual of Japanese Descent
Conclusion
A method was developed for fast, accurate, and precise analysis of vitamin K1 and K2 (MK4 and MK7) in plasma. With quick and easy phospholipid removal sample preparation, 4 minutes of chromatographic analysis using a Raptor Biphenyl column, and sensitive MS/MS detection, the established method provides a reliable and high-throughput assay for clinical research and assessment of vitamin K1 and K2.