Analysis of Terpenes in Cannabis Flower via LI-Syringe-GC-MS
By Colton Myers1, Kelsey Anderson2, Paul Hamrah2, Jason Herrington1
1 Restek Corporation, 2 Verity Analytics
Abstract
The cannabis market in the United States has been growing exponentially as well as starting to emerge in other countries across the globe. Due to expanding legalization, testing demands have greatly increased along with the need for new analytical methodologies. The analysis of terpenes in a variety of commodities has become one of the most popular tests for cannabis laboratories. The results discussed in this research focused on the development of a simple workflow solution for laboratories conducting this analysis. From this research, it was found that a standard liquid injection by syringe (LI-syringe) paired with gas chromatography–mass spectrometry (GC-MS) proved to be a straightforward and robust method for sample introduction and analysis when investigating terpenes. The LI-syringe-GC-MS method achieved a calibration working range of 0.04–5.12 µg/mL; r2 values of 0.988-0.996 (0.993 average); limit of quantification values of 0.017–0.129 µg/mL (0.047 average); analytical precisions of 0.55–9.64% RSD (1.56 average); overall LI-syringe-GC-MS method precisions of 1.73–14.6% RSD (4.97 average); and recoveries of 84.6–98.9% (90.2 average) for the 23 terpenes of interest. Sample workflow and results are discussed.
Introduction
Terpenes are a naturally occurring class of compounds that are found in different species of plants and are typically strong in odor [1]. Previous research has shown that over 100 terpenes have been identified in different cannabis chemical varieties (chemovars) [2]. The analysis of terpenes in cannabis has classically been done via headspace (HS)-GC-FID/MS, often using a full evaporative technique (FET) approach that uses small sample volumes. This works well for monoterpenes, which are smaller, volatile compounds. However, calibrating sesquiterpenes when using HS is more difficult because they are larger and less volatile.
To provide an improved method for sesquiterpenes in cannabis flower, we developed and validated the following workflow using a traditional GC-MS liquid injection approach with a simple accelerated solvent extraction (ASE) sample preparation [3]. A unique aspect of this application is the use of a terpene-free surrogate matrix for matrix-matched calibrations. The advantages of matrix-matched calibrations include, but are not limited to, reducing inaccuracies in reporting and accounting for signal enhancement/suppression caused by matrix interferences.
Experimental
The experiments conducted in this study were designed to develop a fully validated (i.e., validated with the California Bureau of Cannabis Control [BCC]) terpenes method for cannabis flower.
Sample Matrices
Hop pellets were utilized as a terpene-free surrogate matrix for calibration curves; laboratory control samples (LCS); continuing calibration verification (CCV) samples; detection limits; and analytical precision samples. Cannabis shake was used for the LI-syringe-GC-MS method precision and accuracy experiments. Cannabis flower was used for chemovar testing. All matrices were homogenized on a sheet pan with a rolling pin prior to extraction.
Removal of Terpenes from the Hops Surrogate Matrix
Following homogenization, terpenes were removed from the hops surrogate matrix using a proprietary solvent cleaning process that was performed by Verity Analytics. To remove terpenes, homogenized hops can be extracted using either a manual shakeout method or an ASE 350 system. Previous work on the analysis of terpenes in cannabis demonstrated that there was no statistical difference between these procedures for most terpenes extracted from cannabis flower (except for linalool and camphene) [3]. Because the ASE 350 method produces similar results and reduces both labor and the amount of nonreusable materials that are consumed, it was used for the experiments presented here. Once prepared, the extracted hops were placed in an oven to evaporate any remaining solvent. Dried hops were then stored in an airtight container for later use.
Accelerated Solvent Extractor (ASE) Sample Preparation
For all experiments, 0.5 g samples of hops surrogate matrix, cannabis flower, or shake was weighed out and placed in a 10 mL ASE 350 stainless-steel extraction cell (cat.# 25995). The remaining volume in the cell was filled with diatomaceous earth. Samples were extracted using isopropyl alcohol (IPA) using the parameters outlined in Table I. After the samples were extracted, which typically produced 10–11 mL of extract, the extracts were brought to a consistent final volume of 12 mL. Then, 3 mL aliquots of extract were filtered using a Norm-Ject syringe (3 mL Luer Lock tip) (cat.# 22773) with a 0.22 µm PTFE filter (cat.# 26142). For LI-syringe-GC-MS method validation experiments, 500 µL of the filtered extract was added to a 2 mL autosampler vial (cat.# 21142) using a 1 mL gas-tight syringe (cat.# 24575) and then spiked with 5 µL of a 10 µg/mL naphthalene-d8 internal standard (ISTD) solution (cat.# 31043).
Table I: Dionex ASE 350 Extraction Parameters for the Analysis of Terpenes in Cannabis
| Temperature | 75 °C |
| Pressure | 1500 psi |
| Extraction Solvent | Isopropanol (IPA) |
| Static Time | 5 min |
| Purge Time | 90 sec |
| Heat Time | 5 min |
| Cycles | 1 |
Terpene Calibration and ISTD Solution Preparation
Preparation steps for the terpene and ISTD solutions for all experiments are outlined in Table II. After the calibration stock solution was prepared, serial dilutions were made according to Table III. Once calibration levels were prepared, they were spiked with an ISTD solution. For calibration levels 2–10, 5 µL of the 10 µg/mL ISTD were added. For calibration level 1, 10 µL of 10 µg/mL ISTD were added because the final volume of this calibration level was double that of the other levels.
Table II: Terpene Calibration and ISTD Solution Preparation
| Step | Terpene Calibration Solution Preparation (Starting from 2500 µg/mL Cannabis Terpene Standards 1 & 2 [cat.# 34095 & 34096]) |
ISTD Solution Preparation (Starting from 2000 µg/mL Naphthalene-d8 Standard [cat.# 31043]) |
| 1. | Add 400 µL of each terpene standard to 200 µL of IPA (1000 µg/mL stock solution). | Add 50 µL of Naphthalene-d8 standard (ISTD) to 9.95 mL of IPA (final concentration = 10 µg/mL). |
| 2. | Add 61.4 µL of terpene stock solution to cleaned hops in ASE cell. | |
| 3. | Extract using ASE 350 (see Table I). | |
| 4. | Bring extract volume to 12 mL using IPA (final concentration = 5.12 µg/mL). | |
| 5. | Prepare calibration standards by making the serial dilutions outlined in Table III from this extract. |
Table III: Serial Dilutions for Terpene Calibration
| Calibration Level | Concentration (µg/mL) | Amount of Previous Standard (µL) | Amount of IPA (µL) | Final Amount (µL)* |
| 10 | 5.12 | -- | -- | 500 |
| 9 | 2.56 | 500 | 500 | 500 |
| 8 | 1.28 | 500 | 500 | 500 |
| 7 | 0.64 | 500 | 500 | 500 |
| 6 | 0.32 | 500 | 500 | 500 |
| 5 | 0.16 | 500 | 500 | 500 |
| 4 | 0.08 | 500 | 500 | 500 |
| 3 | 0.04 | 500 | 500 | 500 |
| 2 | 0.02 | 500 | 500 | 500 |
| 1 | 0.01 | 500 | 500 | 1000 |
*Final volumes reflect the subsequent transfer of 500 µL to the next standard in the series.
LI-Syringe-GC-MS Calibration and Method Validation
Method validation was completed by analyzing samples to determine terpene calibration; method detection limit (MDL)/limit of quantification (LOQ); analytical precision; method precision; and percent recovery. MDL/LOQ was determined from seven replicate low-level calibration points. LCS were made from the cleaned hop extracts at a concentration equivalent to calibration level 6 (0.32 μg/mL) and run to evaluate analytical precision and percent recovery. LI-syringe-GC-MS method precision was determined from seven different aliquots of cannabis shake extract and three different cannabis flower chemovars were tested to determine terpene content.
GC-MS Method Conditions for the Analysis of Terpenes in Cannabis
See Table IV and Table V for GC-MS selected ion monitoring (SIM) conditions.
Table IV: GC-MS Instrument Parameters
| Thermo Scientific Trace 1310/TSQ 9000 Parameters | |
| Column | Rxi-624Sil MS, 30 m x 0.25 mm x 1.4 µm (cat.# 13868) |
| Injection | Liquid injection (LI-syringe) |
| Inj. Vol. | 1 µL |
| Mode | Split (20:1) |
| Liner | Topaz 4.0 mm Precision liner w/ wool (cat.# 23305) |
| Inj. Temp. | 280 °C |
| Purge Flow | 5 mL/min |
| Oven | 80 °C to 130 °C at 20 °C/min (hold 4 min) to 275 °C at 17 °C/min (hold 0.47 min) |
| Carrier Gas | He, constant flow |
| Flow Rate | 1.5 mL/min |
| Detector/Mode | Single quad/SIM |
| Transfer Line Temp. | 275 °C |
| Ion Source Temp. | 275 °C |
Table V: MS SIM Parameters
| # | Name | Classification | Retention Time (min) | Masses |
| 1 | α-Pinene | monoterpenoid | 4.52 | 91, 92.1, 93.1 |
| 2 | Camphene | monoterpenoid | 4.88 | 79.1, 93.1, 121 |
| 3 | β-Myrcene | monoterpenoid | 5.2 | 93.1 |
| 4 | β-Pinene | monoterpenoid | 5.39 | 79.1, 93.1 |
| 5 | Carene | monoterpenoid | 5.81 | 77, 93.1 |
| 6 | α-Terpinene | monoterpenoid | 5.99 | 77, 93.1 |
| 7 | trans-Ocimene | monoterpenoid | 6.12 | 93.1 |
| 8 | D-Limonene | monoterpenoid | 6.23 | 93.1 |
| 9 | p-Cymene | monoterpenoid | 6.33 | 119 |
| 10 | cis-Ocimene | monoterpenoid | 6.44 | 93.1 |
| 11 | Eucalyptol (1,8-cineole) | monoterpenoid | 6.57 | 81 |
| 12 | γ-Terpinene | monoterpenoid | 6.87 | 91, 93.1 |
| 13 | Terpinolene | monoterpenoid | 7.49 | 91, 93.1 |
| 14 | Linalool | monoterpenoid | 8.16 | 91, 92.1, 93.1 |
| 15 | Isopulegol | monoterpenoid | 9.19 | 67, 69.1 |
| 16 | Naphthalene-d8 (ISTD) | NA | 9.83 | 136 |
| 17 | Geraniol | monoterpenoid | 10.51 | 67.1, 69.1 |
| 18 | β-Caryophyllene | sesquiterpenoid | 12.19 | 91, 93.1 |
| 19 | α-Humulene | sesquiterpenoid | 12.56 | 91, 92.1, 93.1 |
| 20 | cis-Nerolidol | sesquiterpenoid | 13.15 | 79.1, 91, 93.1 |
| 21 | trans-Nerolidol | sesquiterpenoid | 13.42 | 79.1, 91, 93.1 |
| 22 | Guaiol | sesquiterpenoid | 13.95 | 93.1, 161 |
| 23 | (-)-Caryophyllene oxide | sesquiterpenoid oxide | 14.12 | 69.1, 79.1, 93.1 |
| 24 | α-Bisabolol | sesquiterpenoid | 14.52 | 93.1, 119 |
Results and Discussion
LI-Syringe-GS-MS Method Verification
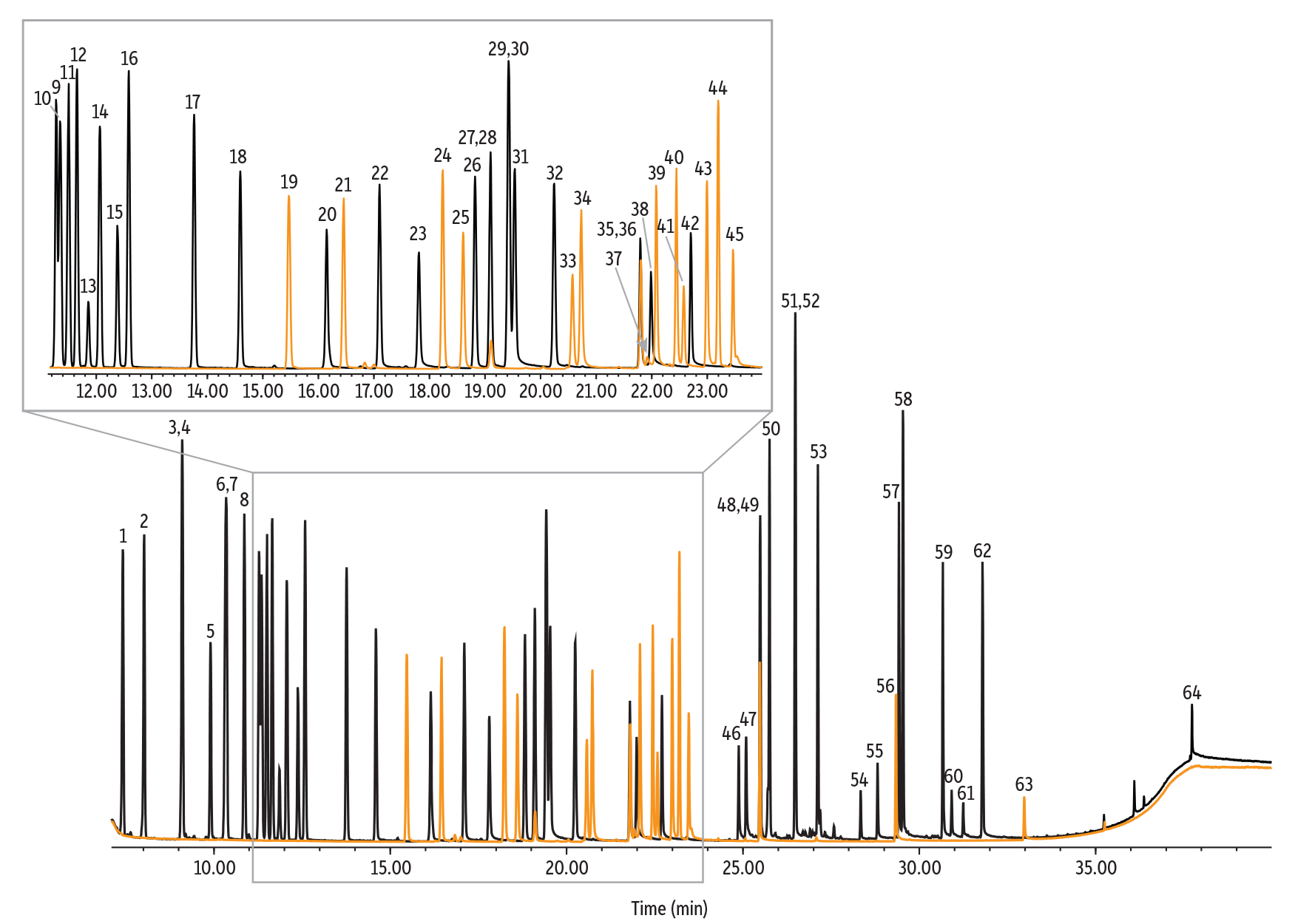
As shown in Figure 1, all 23 target compounds were separated on an Rxi-624Sil MS column using the method developed here for the analysis of terpenes in cannabis. Method performance can be seen in Table VI. Over the calibration range of 0.04–5.12 µg/mL, an average r2 value of 0.993 was achieved for the 23 terpenes of interest. Two terpenes (i.e., α-bisabolol and β-caryophyllene) had the shortest calibration range spanning from 0.16–5.12 µg/mL, but maintained good r2 values of 0.990 and 0.992, respectively. An average LOQ of 0.047 µg/mL was achieved as well as average analytical and method precision %RSDs of 1.56% and 4.97% respectively. Percent recoveries for all 23 terpenes met acceptance criteria (±30%) and ranged from 84.6% to 98.9% with an average of 90.2%.
Figure 1: Chromatographic Separation of 23 Terpenes
|
|
Table VI: LI-Syringe-GC-MS Method Validation
| Compound | Retention Time (min) | Working Range (µg/mL) | r² | LOQ (µg/mL) | Analytical Precision (%RSD) | Method Precision (%RSD) | Recovery (%) |
| α-Pinene | 4.263 | 0.08 - 5.12 | 0.994 | 0.041 | 1.36 | 8.52 | 93.6 |
| Camphene | 4.581 | 0.04 - 5.12 | 0.996 | 0.038 | 1.25 | 5.17 | 93.6 |
| β-Myrcene | 4.928 | 0.08 - 5.12 | 0.992 | 0.046 | 1.51 | 3.17 | 91.6 |
| β-Pinene | 5.020 | 0.08 - 5.12 | 0.998 | 0.042 | 1.40 | 6.20 | 88.8 |
| Carene | 5.456 | 0.08 - 5.12 | 0.995 | 0.057 | 1.90 | 4.84 | 91.2 |
| α-Terpinene | 5.629 | 0.04 - 5.12 | 0.992 | 0.036 | 1.19 | 5.56 | 88.0 |
| trans-Ocimene | 5.736 | 0.04 - 5.12 | 0.995 | 0.022 | 0.71 | 3.35 | 88.8 |
| D-Limonene | 5.851 | 0.04 - 5.12 | 0.994 | 0.027 | 0.91 | 5.25 | 88.8 |
| p-Cymene | 5.908 | 0.04 - 5.12 | 0.994 | 0.040 | 1.32 | ND | 88.9 |
| cis-Ocimene | 6.053 | 0.02 - 5.12 | 0.993 | 0.017 | 0.55 | 3.04 | 88.4 |
| Eucalyptol (1,8-Cineole) | 6.142 | 0.04 - 5.12 | 0.996 | 0.032 | 1.07 | 9.04 | 91.2 |
| γ-Terpinene | 6.440 | 0.04 - 5.12 | 0.993 | 0.033 | 1.09 | 6.51 | 88.7 |
| Terpinolene | 7.112 | 0.04 - 5.12 | 0.993 | 0.035 | 1.16 | 4.35 | 90.1 |
| Linalool | 7.795 | 0.08 - 5.12 | 0.991 | 0.076 | 2.53 | 2.99 | 90.2 |
| Isopulegol | 8.848 | 0.04 - 5.12 | 0.993 | 0.029 | 0.95 | 3.31 | 90.2 |
| Naphthalene-d8 (ISTD) | 9.531 | NA | NA | NA | NA | NA | NA |
| Geraniol | 10.218 | 0.04 - 5.12 | 0.988 | 0.039 | 1.29 | 3.31 | 88.2 |
| β-Caryophyllene | 12.042 | 0.16 - 5.12 | 0.992 | 0.129 | 4.27 | 3.90 | 91.6 |
| α-Humulene | 12.384 | 0.04 - 5.12 | 0.991 | 0.027 | 0.91 | 3.88 | 86.7 |
| cis-Nerolidol | 12.940 | 0.08 - 5.12 | 0.994 | 0.080 | 2.67 | 14.57 | 89.3 |
| trans-Nerolidol | 13.192 | 0.08 - 5.12 | 0.989 | 0.050 | 1.65 | 2.58 | 98.9 |
| Guaiol | 13.720 | 0.08 - 5.12 | 0.993 | 0.060 | 1.98 | 3.27 | 85.9 |
| (-)-Caryophyllene Oxide | 13.816 | 0.04 - 5.12 | 0.993 | 0.030 | 0.98 | 1.73 | 84.6 |
| α-Bisabolol | 14.296 | 0.16 - 5.12 | 0.990 | 0.094 | 3.11 | 4.82 | 96.1 |
| Average | 0.993 | 0.047 | 1.56 | 4.97 | 90.2 |
ND – Not Detected
NA – Not Applicable
Analysis of Terpenes in Cannabis Flower
Once the LI-syringe-GC-MS method was established, the cannabis chemovar “mint chocolate chip” was tested for terpene content. The aforementioned workflow was applied to this cannabis flower sample, and the results, corrected for ASE extraction and dilution, are shown in Table VII. Of the 23 terpenes analyzed, 22 were found in the mint chocolate chip chemovar. p-Cymene was not detected (ND) in the sample, and two terpenes of interest (carene and guaiol) were reported at concentrations that fell outside of their calibration ranges.
Table VII: Mint Chocolate Chip Chemovar Terpene Results
| # | Compound | LI-Syringe-GC-MS (µg/g) |
| 1 | α-Pinene | 32.2 |
| 2 | Camphene | 3.84 |
| 3 | β-Myrcene | 8.24 |
| 4 | β-Pinene | 25.7 |
| 5 | Carene* | 1.59 |
| 6 | α-Terpinene | 1.95 |
| 7 | trans-Ocimene | 2.02 |
| 8 | D-Limonene | 52.1 |
| 9 | p-Cymene | ND |
| 10 | cis-Ocimene | 4.55 |
| 11 | Eucalyptol (1,8-Cineole) | 1.46 |
| 12 | γ-Terpinene | 1.97 |
| 13 | Terpinolene | 2.21 |
| 14 | Linalool | 106 |
| 15 | Isopulegol | 3.96 |
| 16 | Naphthalene-d8 (ISTD) | NA |
| 17 | Geraniol | 4.45 |
| 18 | β-Caryophyllene | 45.6 |
| 19 | α-Humulene | 14.8 |
| 20 | cis-Nerolidol | 25.4 |
| 21 | trans-Nerolidol | 9.46 |
| 22 | Guaiol* | 1.61 |
| 23 | (-)-Caryophyllene Oxide | 2.97 |
| 24 | α-Bisabolol | 14.7 |
| Average | 16.7 |
*Concentration outside of calibration range for LI-Syringe-GC-MS
Figure 2 is a graphical representation of the most abundant terpenes found in the mint chocolate chip chemovar. Linalool was reported with the highest concentration (106 µg/g) making up 29% of the total terpene content. Other terpenes at higher percentages include D-limonene (14%), β-caryophyllene (12%), α-pinene (9%), and β-pinene (7%).
Figure 2: Most Abundant Terpenes Identified in Mint Chocolate Chip Chemovar by LI-Syringe-GC-MS
Conclusion
A method was developed for the analysis of terpenes in cannabis flower via LI-syringe-GC-MS using SIM. This workflow provided good results for both monoterpenes and the more challenging sesquiterpenes as demonstrated by an average r2 value of 0.993; LOQ of 0.047 µg/mL; analytical precision of 1.56 %RSD; method precision of 4.97 %RSD; and recovery of 90.2%. It should be noted that this workflow used accelerated solvent extraction to reduce labor and materials, but previous work demonstrated that hand-shakeout extractions showed negligible differences and can also be applied to this method [3]. Additionally, this work included the analysis of terpenes in cannabis flower extracts because there is great interest in this area. Finally, when testing cannabis products, careful consideration should be given to utilizing matrix-matched calibrations as was done here to mitigate signal enhancement/suppression caused by matrix effects.
Additional Considerations
Since the development of this workflow, additional work was done to expand the number of terpenes that could be tested in cannabis and similar matrices. The current sample preparation workflow is anticipated to be suitable for the expanded terpenes list, but interested labs should verify this independently and note that the analytical methods use different GC columns and conditions. The expanded terpenes analysis uses an Rxi-1301Sil MS column (cat.# 16094) and terpene MegaMix #1 & 2 standards (cat.# 34142 & 34143). Chromatographic separation and conditions for the expanded list are shown in Figure 3.
Figure 3: Expanded Terpenes List on an Rxi-1301Sil MS Column
| Column | Rxi-1301Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 16094) |
|---|---|
| Standard/Sample | Terpenes MegaMix #1 (cat.# 34142) |
| Terpenes MegaMix #2 (cat.# 34143) | |
| Diluent: | Isopropanol |
| Conc.: | 10 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 25:1) |
| Liner: | Topaz 4.0 mm ID Precision inlet liner w/ wool (cat.# 23305) |
| Inj. Temp.: | 280 °C |
| Split Vent Flow Rate: | 45 mL/min |
| Oven | |
| Oven Temp.: | 40 °C (hold 0.5 min) to 100 °C at 3 °C/min to 200 °C at 8 °C/min to 300 °C at 25 °C/min (hold 3 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.8 mL/min |
| Detector | 5977B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 300 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | HES | ||||||||
| Source Temp.: | 325 °C | ||||||||
| Quad Temp.: | 200 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Solvent Delay Time: | 6.5 min | ||||||||
| Tune Type: | BFB | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977B MSD | ||||||||
References
- T. Nutinnen, Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus, European Journal of Medicinal Chemistry, 157 (2018) 198-228. https://doi.org/10.1016/j.ejmech.2018.07.076
- L. Calvi, D. Pentimalli, S. Panseri, L. Giupponi, F. Gelmini, G. Beretta, D. Vitali, M. Bruno, E. Zilio, R. Pavlovic, A. Giorgi, Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap) approach, Journal of Pharmaceutical and Biomedical Analysis, 150 (2018) 208-219. https://doi.org/10.1016/j.jpba.2017.11.073
- C. Myers, J.S. Herrington, P. Hamrah, K. Anderson, Accelerated solvent extraction of terpenes in cannabis coupled with various injection techniques for GC-MS analysis, Frontiers in Chemistry, 9 (2021) 1-13. https://doi.org/10.3389/fchem.2021.619770

