Comprehensive LC-MS/MS Analysis of 15 Bisphenols in 8 Minutes
Featured Application: Bisphenols on Raptor Biphenyl
- Excellent peak shape and separation for bisphenol A and common analogues.
- Simple, no-additives mobile phases and gradient program.
- Fast, 8-minute total cycle time.
Bisphenol A (BPA), widely used in the production of polycarbonate plastic and epoxy resins, is an endocrine disruptor that imitates naturally occurring hormones or acts as an antagonist, both of which can cause harmful effects on hormone biosynthesis, metabolism, distribution, and mode of action. Hormone disruption can result in detrimental effects to health, growth, and reproduction. Children and unborn babies are most susceptible to hormonal and neurological development problems, and the prevalent use of BPA in many consumer products, including food and beverage packaging, adhesives, and toys, has caused several governments to investigate its safety. Due to the wide range of exposure routes and vulnerability of children, negative public perception has driven many products to advertise as "BPA-free," instead opting to use BPA analogues that have similar physicochemical properties. However, these alternate bisphenols are understudied and may also have harmful toxicological profiles. Some research has shown that these compounds, notably BPF, BPS, BPAF, BPZ, BPE, and BPB, are estrogenic endocrine disruptors and may cause health effects similar to BPA [1,2].
Establishing accurate methods for the analysis of bisphenols that include a broad suite of compounds, rather than just BPA, is imperative for both investigating toxicology and monitoring human exposure. The simple LC-MS/MS gradient method presented here was developed on a Raptor Biphenyl column because it provides excellent chromatographic peak shape and baseline separation of 15 bisphenols, including BPA and its most prevalent analogues. The 1.8 µm, 2.1 x 50 column format takes advantage of the inherent speed gains of small particle size technology, resulting in a fast, 8-minute analysis that is ideal for high-throughput testing.
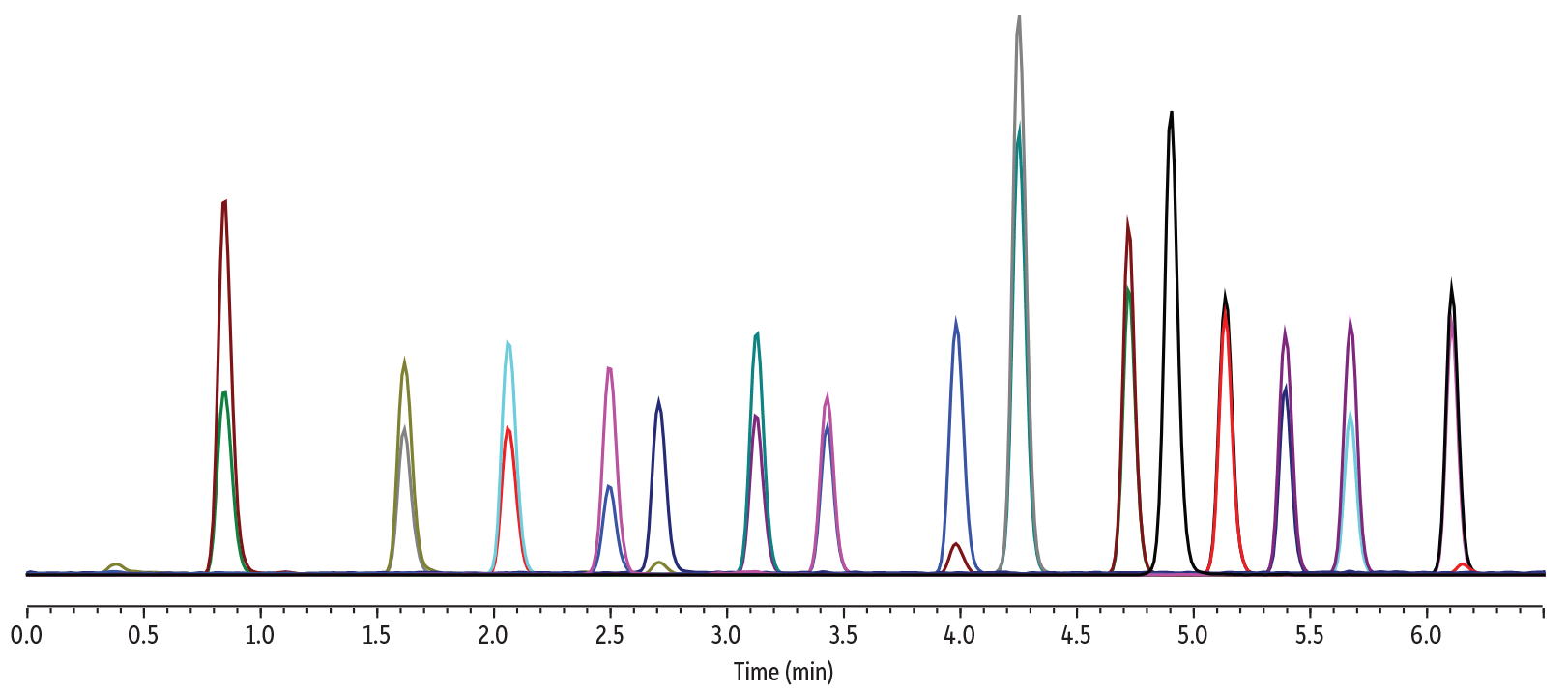
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Bisphenol S | 0.84 | 5.00 | 249.2 | 108.1 | 92.1 |
| 2. | Bisphenol F | 1.62 | 350 | 199.3 | 93.1 | 105.1 |
| 3. | Bisphenol E | 2.06 | 100 | 213.3 | 198.3 | 197.4 |
| 4. | Bisphenol A | 2.50 | 100 | 227.3 | 212.3 | 133.1 |
| 5. | Bisphenol AF | 2.71 | 2.00 | 335.2 | 265.3 | 177.3 |
| 6. | Bisphenol B | 3.13 | 100 | 241.3 | 212.4 | 211.3 |
| 7. | Bisphenol C | 3.43 | 350 | 255.3 | 240.4 | 147.3 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 8. | Bisphenol AP | 3.98 | 25.0 | 289.3 | 274.3 | 273.3 |
| 9. | Bisphenol Z | 4.25 | 250 | 267.2 | 173.4 | 145.2 |
| 10. | Bisphenol G | 4.72 | 250 | 311.2 | 295.4 | 296.4 |
| 11. | Bisphenol FL | 4.90 | 50.0 | 348.8 | 256.2 | - |
| 12. | Bisphenol BP | 5.14 | 50.0 | 351.2 | 273.3 | 274.3 |
| 13. | Bisphenol M | 5.39 | 15.0 | 345.2 | 330.3 | 251.4 |
| 14. | Bisphenol P | 5.67 | 50.0 | 345.2 | 330.4 | 315.3 |
| 15. | Bisphenol PH | 6.11 | 350 | 379.2 | 209.4 | 364.4 |
| Column | Raptor Biphenyl (cat.# 9309252) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 1.8 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 25 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 75:25 Water:methanol | ||||||||||||||||||||
| Conc.: | 2.00-350 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water | ||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
References
[1] R. Mesnage, A. Phedonos, M. Arno, S. Balu, J.C. Corton, M.N. Antoniou, Transcriptome profiling reveals bisphenol A alternatives activate estrogen receptor alpha in human breast cancer cells. Toxicol. Sci. 158 (2) (2017) 431-443. https://doi.org/10.1093/toxsci/kfx101
[2] R. Barouki, E. Tarroja, C. Persoz, C. Thomsen, E. Heyvaert, H. Reyders, K. Van Campenhout, G. Schoeters, C. Ganzleben, C. Hartmann, M. Uhl, J.-P. Antignac, L. Debrauwer, N. Janey Holcer, N. Cingotti, N. Reineke, Scoping documents: prioritized substance group: bisphenols. HBM4EU (2018) 1-21. https://www.hbm4eu.eu/wp-content/uploads/2017/04/scoping-document-on-bisphenols.pdf

