Protect Natural Gas Sample Integrity and Prevent Sulfur Loss with Sulfinert Sample Cylinders
Determining the sulfur content of natural gas is critical as sulfur containing compounds can damage equipment and reduce BTU values. However accurate quantification is challenging due to adsorption in sample collection devices. Sulfinert sample cylinders are significantly more inert than stainless steel cylinders and assure sample integrity during collection, transportation, and storage.
Accurate analysis of sulfur compounds in refinery feedstocks, process plant streams, and gas fuels delivered by pipeline has become increasingly important as fuel markets expand. Naturally occurring sulfurs in fuel gas adversely impact processing, delivery, and utilization by corroding equipment, poisoning catalysts, and reducing BTU values. Sulfur compounds that are added to liquefied natural gas (LNG) and other fuels as odorants for safety purposes can also have these negative effects. In all cases, accurate sulfur analysis is critical for equipment and pipeline protection. Since sulfur compounds are highly reactive, analytical accuracy depends heavily on the inertness of the sampling and storage equipment.
Sulfur containing compounds, such as hydrogen sulfide, mercaptans, and thiophenes, are extremely reactive with stainless steel surfaces; they are easily adsorbed and show losses within minutes or hours. In contrast, sample cylinders passivated with a Sulfinert coating show very low reactivity and are ideal for collecting, transporting, and storing fuel gases containing these compounds. The Sulfinert surface, consisting of a chemically modified silicon layer, provides the inertness needed for monitoring highly-reactive sulfur compounds, down to ppbv levels.
Sulfinert Treatment Prevents the Loss of Sulfur Compounds
To demonstrate the protective effect of a Sulfinert coating, we collected natural gas samples at the point of use and compared sulfur compound stability over time. One sample was collected in a 1 L stainless steel sample cylinder with stainless steel valves at each end. The second sample was collected in a 1 L Sulfinert sample cylinder with Sulfinert valves at each end. Both samples were collected simultaneously and were analyzed within 30 minutes to establish a baseline. The samples were analyzed by GC using an Rtx-1 column (100% polydimethylsiloxane) and a sulfur chemiluminescence detector. All fittings and transfer lines from the sample cylinder to the sample loop were Sulfinert-passivated to minimize sulfur adsorption. To determine losses, ethyl mercaptan was monitored over time using dimethyl sulfide, a stable compound already contained in natural gas, as an internal standard.
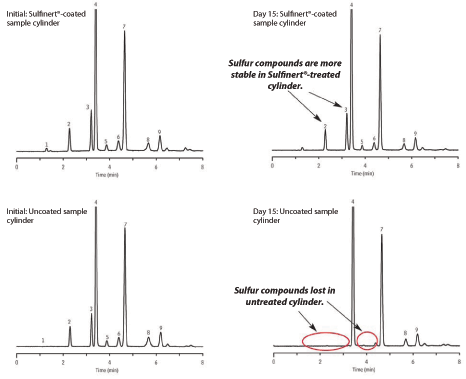
Samples tested within 30 minutes of collection were quite comparable, but sulfur losses were quickly evident in the stainless steel cylinder. Within 24 hours, 34% of the ethyl mercaptan was lost from the stainless steel sample cylinder (Figure 1). This increased to 56% by Day 4, compared to just 6% from the Sulfinert-treated cylinder during the same test period. Sample integrity was completely compromised by Day 16 in the stainless steel cylinder, when no traces of ethyl mercaptan were detected. In addition, methyl mercaptan, 2-propanethiol, and 1-propanethiol were completely lost in the stainless steel cylinder, but remained present in the Sulfinert sample cylinder in the same timeframe (Figure 2). Sulfur losses were minimal and compounds were still quantifiable in a Sulfinert sample cylinder, long after they were completely adsorbed in the non-passivated, stainless steel sample cylinder.
Summary
Cylinders passivated with a Sulfinert coating are ideal for collecting, transporting, and storing fuel gases containing reactive sulfurs. The Sulfinert coating minimizes adsorption, ensuring analyte stability and analytical results that accurately reflect true values for reactive sulfurs in natural gas process streams and finished product.
Figure 1: Samples collected in Sulfinert treated cylinders are significantly more stable than those collected in untreated cylinders. Samples in untreated cylinders quickly lose reactive sulfurs, due to interaction with the stainless steel surface.
Figure 2: Using Sulfinert treated sample cylinders prevents the loss of sulfur compounds and assures samples are representative of their source. Samples collected in untreated stainless steel cylinders do not maintain their integrity during typical storage periods.
| Peaks | Concentration (ppbv) | |
|---|---|---|
| 1. | Hydrogen sulfide | 20 |
| 2. | Methyl mercaptan | 200 |
| 3. | Ethyl mercaptan | 300 |
| 4. | Dimethyl sulfide | 2,000 |
| 5. | 2-Propanethiol | 50 |
| 6. | 1-Propanethiol | 100 |
| 7. | Ethyl methyl sulfide | 1,300 |
| 8. | 2-Butanethiol | 150 |
| 9. | Diethyl sulfide | 180 |
| Column | Rtx-1, 60 m, 0.53 mm ID, 7.00 µm (cat.# 10193) |
|---|---|
| Standard/Sample | Natural gas |
| Injection | Direct |
| Inj. Temp.: | 30 °C |
| Oven | |
| Oven Temp.: | 30 °C (hold 1 min) to 100 °C at 30 °C/min (hold 60 min) |
| Carrier Gas | He, constant pressure |
| Linear Velocity: | 96 cm/sec @ 30 °C |
| Detector | Sievers 355 SCD @ 800 °C |
|---|---|
| Hydrogen Flow Rate: | 100 mL/min |
| Air Flow Rate: | 40 mL/min |
| Instrument | HP5890 GC |
| Notes | Point of use samples were collected in either an uncoated sampling cylinder or a Sulfinert-treated sampling cylinder. A Valco sampling valve with a 1 mL external loop was used. |
