Revive In-Line Sample Preparation (ILSP): A Faster Approach for Multiresidue Pesticides in Food
- Automated, in-line sample extract cleanup dramatically reduces sample preparation time.
- Simultaneous analysis and ILSP cartridge wash eliminate downtime between samples.
- Fast, simple alternative to QuEChERS or SPE for multiresidue pesticides analysis in foods.
In-line sample preparation (ILSP) is an ideal sample preparation technique for food safety labs seeking to spend less time and money per LC-MS/MS sample without sacrificing performance. A Revive ILSP Pesticides cartridge separates analytes from potentially interfering matrix components just like conventional SPE and QuEChERS methods do, but it replaces time-consuming manual procedures with an automated cleanup process that occurs on the instrument in line with the analytical sample flow path. Because Revive ILSP Pesticides cartridges leverage effective retention mechanisms (e.g., reversed-phase interactions) and efficient particle design, they provide a powerful, automated, chromatographic cleanup of complex samples that occurs on the instrument concurrent with sample analysis.
As an example of the benefits of in-line sample preparation, Figure 1 compares a Revive ILSP Pesticides workflow and a typical QuEChERS workflow for the analysis of pesticide residues in spinach. The ISLP approach resulted in a time savings of 1.5 hours with fewer sample transfers, providing a significant leap forward in sample preparation productivity. ILSP also minimizes the risk of introducing error through manual steps and provides equivalent or superior analytical results compared to QuEChERS. An exhaustive study of this comparison has been published by Lupo, et al. [1].
Figure 1: Revive in-line sample preparation is 1.5 hours faster than a traditional QuEChERS workflow for 14 spinach samples.
How Revive In-Line Sample Preparation Works
As shown in Figure 2, Revive ILSP incorporates a sample cleanup cartridge directly into the LC injection flow path. Once the analytes reach the analytical column, the ILSP cleanup cartridge is then backflushed or “revived,” preparing it for the next injection while the first sample is analyzed. Concurrent regeneration and analysis are made possible using a six-port valve and a standard independent isocratic pump. Detailed guidance on instrument configuration, setting method parameters (e.g., valve timing and rinse solvent flow rate), and identifying an effective rinse solvent are given in Restek’s ILSP Method Development Guidelines document. The day-to-day savings in operating costs will quickly pay for the investment in a pump and time spent setting up the method, especially for labs with high volumes of samples or limited sample preparation resources.
Figure 2: How In-Line Sample Preparation Works
Consistent, Cost-Effective Results Over Long Lifetimes
Beyond time savings, a lifetime experiment conducted using 300 injections of avocado extracts provides an excellent example of the consistent results and substantial cost savings that ILSP also offers. Avocado, a high-fat commodity, was fortified with a representative mix of 61 pesticides that ranged in chemical characteristics and retention times. Samples were then extracted and analyzed using the optimized workflow shown in Figure 3. The in-line sample preparation method was significantly faster than a comparable QuEChERS avocado workflow, and a single Revive ILSP Pesticides cartridge provided effective cleanup and consistent chromatographic results over the duration of the experiment.
Figure 3: An example in-line sample preparation workflow for high-fat avocado samples.
The three pesticides highlighted in Figure 4 represent early, mid, and late-eluting compounds from across the entire analyte list. Consistent retention times, peak shapes, and responses over the course of hundreds of matrix injections demonstrate the robustness of Revive ILSP Pesticides cartridge cleanup. Of the 61 pesticides monitored, 95% met the performance guidelines established by the European Union Reference Laboratories’ SANTE/12682/2019 guidelines (70-120% recoveries, %RSD ≤ 20%) when evaluated as a series of triplicate injections of a single extract [2].
In addition to speed gains and robust performance, ILSP also offers significant cost savings. For example, if we assume a $4 per sample cost for each QuEChERS sample (extraction salts packet and dSPE tube), the 300 avocado samples would have cost $1200 in QuEChERS products alone. The Revive in-line sample preparation workflow uses a simple liquid-solid extraction without extraction salts or dSPE, which eliminates that cost. A single Revive ILSP Pesticides cartridge, which costs approximately a quarter of the total QuEChERS price, effectively cleaned the 300 avocado samples, and since its performance had not degraded after 300 injections, replacing the cartridge was not necessary when the experiment ended.
Figure 4: Consistent chromatography even after 300 avocado matrix injections on a single Revive ILSP cartridge.
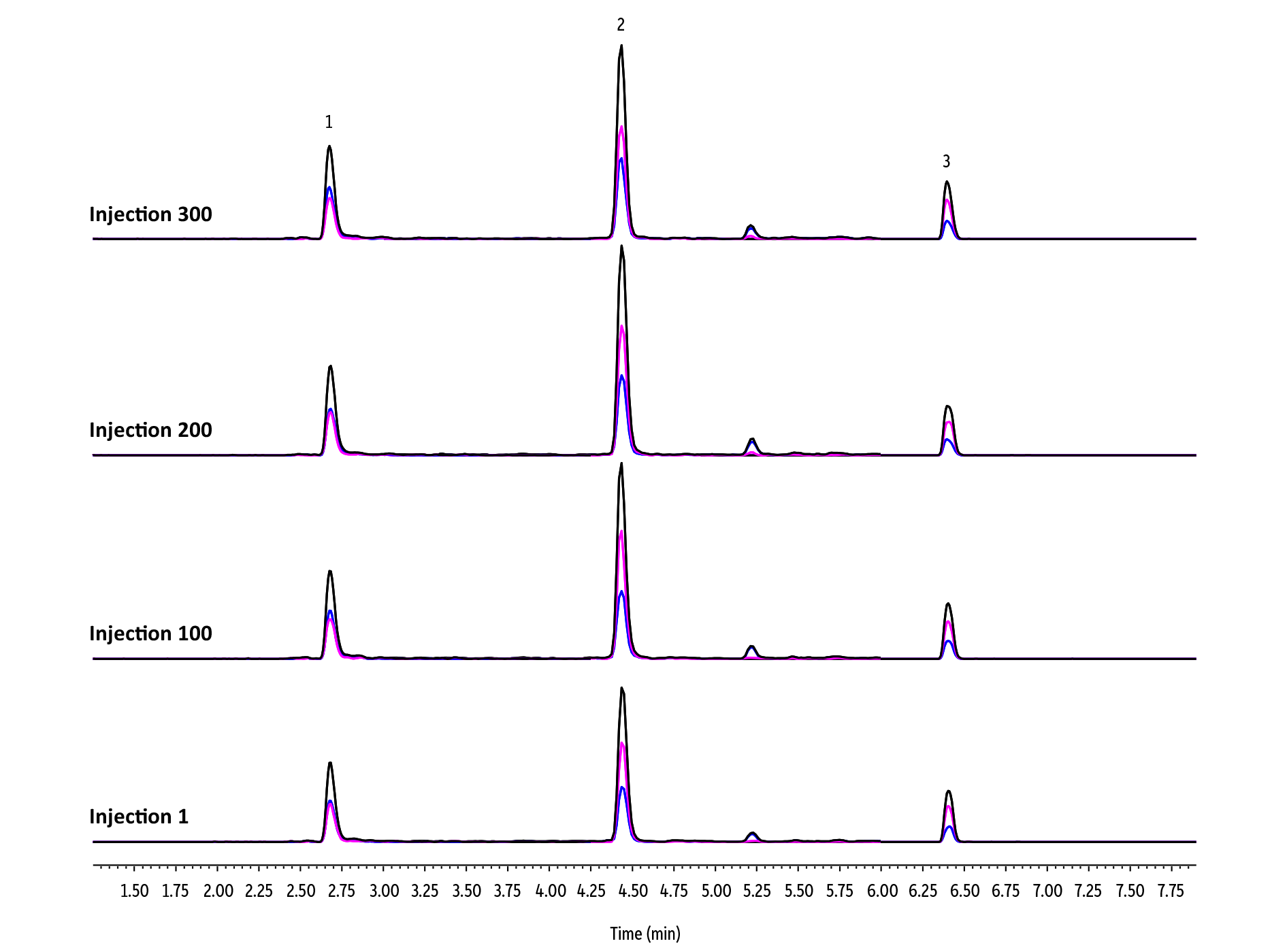
| Peaks | tR (min) | Conc. (ng/g) | Precursor Ion | Product Ion 1 | Product Ion 2 | |
|---|---|---|---|---|---|---|
| 1. | Imidacloprid | 2.678 | 50 | 256.1 | 175.0 | 209.0 |
| 2. | Fenhexamid | 4.557 | 50 | 302.1 | 97.1 | 55.05 |
| 3. | Eprinomectin | 6.396 | 50 | 914.6 | 186.1 | 154.1 |
| Column | Raptor ARC-18 (cat.# 9314A12) | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||||||||||
| Guard Column: | Raptor ARC-18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9314A0252) | ||||||||||||||||||||||||||||||||
| Temp.: | 50 °C | ||||||||||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||||||||||
| Diluent: | Acetonitrile, 0.1% acetic acid | ||||||||||||||||||||||||||||||||
| Inj. Vol.: | 3 µL | ||||||||||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||||||||||
| A: | Water, 0.2% formic acid, 2 mM ammonium formate | ||||||||||||||||||||||||||||||||
| B: | Methanol, 0.2% formic acid, 2 mM ammonium formate | ||||||||||||||||||||||||||||||||
| C: | Methanol, 0.2% formic acid, 10 mM ammonium formate | ||||||||||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
| Sample Preparation | Sample Fortification and Extraction Avocado was peeled and homogenized, and 5 g of sample was weighed into a 50 mL polypropylene tube. 10 mL of acetonitrile containing 0.1% acetic acid was added to the sample and vortexed. Samples were shaken on a shaker table for 10 minutes and then centrifuged at 4200 rpm for 10 minutes. An aliquot was transferred to a vial and fortified with analytes for a final concentration of 50 ng/g. The sample was vortexed, and an aliquot transferred to a 0.2 μm PTFE Thomson filter vial cat.# 25893 and filtered prior to injection. In-Line Sample Preparation (ILSP) The UHPLC system was equipped with an auxiliary pump; 6-port, high-pressure switching valve; and dual-directional 5 x 2.1 mm Revive ILSP Pesticides cartridge and holder (cat.# 27882). At 5.5 min, after the target compounds had all eluted from the ILSP cartridge and were undergoing analysis, valve switching was used as described below to flush the ILSP cartridge and wash trapped matrix components to waste. At 7.0 min, the original valve configuration, where the ILSP cartridge is in-line with the analytical column, was restored and the system was brought back to equilibrium prior to the next injection. • 0 min; valve position 0 • 0 min; C flow= 0 mL/min • 5.49 min; C flow= 0 mL/min • 5.5 min; valve position 1 • 5.5 min; C flow= 1 mL/min • 6.9 min; C flow=1 mL/min • 7 min; C flow= 0 mL/min • 7 min; valve position 0 |
| Notes | Thomson SINGLE StEP standard filter vials cat.# 25893 were used to produce this chromatogram, but have since been discontinued. For assistance choosing a replacement for this application, contact Restek Technical Service or your local Restek representative. |
Revive ILSP in Action: Multiresidue Pesticide Analysis in Disparate Foods
Analyzing pesticides in food commodities is particularly challenging due to the wide range of analyte chemistries and matrix types (high fat, high sugar, high pigmentation, low water, etc.) To address this, a variety of QuEChERS products have been formulated to ensure effective extraction and cleanup for different situations. However, a single Revive ILSP Pesticides cartridge can provide excellent results across the same wide range of analytes and matrices.
To demonstrate this broad applicability, a recovery study was performed using in-line sample preparation methods that were developed for six commodities spanning five of the commodity groups in the SANTE/12682/2019 guidelines (Table I). These foods were fortified with 61 pesticides representing a variety of compound chemistries that elute at different points (early, mid, late) across a typical multiresidue pesticide screening method chromatogram (Table II).
Table I: Commodities used to develop Revive ILSP methods.
|
Commodity Group |
Food Sample |
|
High water and high pigment content |
Spinach |
|
High acid content and high water content |
Whole orange |
|
High lipid content and very low water content |
Soybean meal |
|
High lipid content and intermediate water content |
Avocado |
| "Difficult or unique commodities" | Hibiscus tea |
| Black tea |
Table II: Revive ILSP recovery experiments were performed using 61 pesticides that differed in both chemical characteristics and elution time (early, mid, late) in typical multiresidue pesticide screening methods.
| List of Monitored Pesticides | ||
|
Cyromazine |
Flutolanil |
Benzoximate |
|
Dinotefuran |
Mepronil |
Trifloxystrobin |
|
Nitenpyram |
Myclobutanil |
Metaflumizone |
|
Imidacloprid |
Methoxyfenozide |
Fluazinam |
|
Acetamiprid |
Triadimefon |
Tebufenpyrad |
|
Oxadixyl |
Mepanipyrim |
Pyriproxyfen |
|
Carbetamide |
Fluoxastrobin |
Piperonyl Butoxide |
|
Pyracarbolid |
Fenhexamid |
Quinoxyfen |
|
Secbumeton |
Butafenacil |
Amitraz |
|
Prometon |
Cyprodinil |
Fenpyroximate |
|
Terbumeton |
Picoxystrobin |
Eprinomectin |
|
Ametryn |
Rotenone |
Abamectin B1a |
|
Metalaxyl |
Tebufenozide |
Fenazaquin |
|
Chlorantraniliprole |
Dimoxystrobin |
Doramectin |
|
Pyrimethanil |
Carfentrazone-ethyl +NH4 |
Ivermectin |
|
Spiroxamine |
Kresoxym-methyl |
Moxidectin |
|
Azoxystrobin |
Zoxamide |
Imazalil |
|
Halofenozide |
Famoxadone |
Pymetrozine |
|
Furalaxyl |
Benalaxyl |
Fludioxinol |
|
Boscalid |
Clofentezine |
|
|
Mandipropamid |
Prochloraz |
|
ILSP Sample Extraction
For Revive ILSP extraction, sample homogenization followed by a liquid-solid extraction worked well for all commodities studied. The exact extraction parameters (solvent, volume, shake time, filtration, etc.) were optimized for each commodity, but the overall procedure was simple, quick, and effective: homogenization, solvent addition, agitation, followed by sample filtration, if necessary. In addition, because there is no dispersive cleanup step as is typical of QuEChERS methods, in-line sample preparation methods minimize analyte loss during cleanup.
ILSP Recoveries Across Various Commodity Group
Nearly all of the pesticides studied were recovered from all six different matrices within the performance guidelines provided by SANTE/12682/2019 using an LC-MS/MS with low-to-mid range sensitivity (Table III). These results demonstrate the broad applicability of Revive in-line sample preparation for multiresidue pesticide monitoring in food and feed commodities.
In addition, when Revive ILSP was compared to analogous QuEChERS methods, ILSP performed comparably, if not better, in all cases. And, for instances when compounds fell outside the ideal 70-120% recovery range, guidelines like SANTE/12682/2019 provide provisions for reporting that data, if the results are consistent, as is the case with ILSP’s automated and reproducible cleanup.
Table III: Total recovery performance for Revive ILSP methods developed for six disparate food commodities.
|
Commodity |
Percentage of Compounds with 70-120% Recovery & ≤ 20% RSD (Concentration) |
|
Spinach |
85.7% (5 ng/g); 95.2% (100 ng/g) |
|
Whole Orange |
87% (10 ng/g) |
|
Soybean Meal |
97% (10 ng/g) |
|
Avocado |
95% (10 ng/g) |
|
Hibiscus Tea |
92% (10 ng/g) |
|
Black Tea |
98% (10 ng/g) |
Matrix Effects and ILSP
As in any analysis of real-world samples, some matrix components will likely be extracted along with the target analytes. In this regard, ILSP is no different than any other sample preparation technique and using matrix-matched calibration standards is the best way to assure accurate quantitation. This is particularly true for compounds that elute early in the run where unretained matrix components are most likely to appear and cause enhancement or suppression.
In addition to affecting quantitation, matrix components can contaminate instruments. With in-line sample preparation methods, uncleaned extracts are being injected, so it is important to ensure that internal and external needle rinses are performed between injections to avoid carryover between samples and prevent buildup that could require instrument downtime to remove.
Revive In-Line Sample Preparation: A Faster Approach for Multiresidue Pesticides in Food
In-line sample preparation uses the power of your LC-MS/MS to streamline and automate sample extract cleanup. With a Revive ILSP Pesticides cartridge, six-port valve, and an independent isocratic pump, your instrument can be transformed into an analytical workhorse, combining sample cleanup and analysis in a single, efficient method. Decrease sample preparation time, cut the costs associated with disposable sample prep products, and reduce errors related to manual procedures by integrating Revive ILSP into your current methods for multiresidue pesticide analysis in foods.
References
- A. Lupo, R.L. Romesberg, X. Lu, Automated inline pigment removal for the analysis of pesticide residues in spinach by liquid chromatography tandem mass spectrometry, J. Chromatogr. A 1629 (2020) 461477. https://doi.org/10.1016/j.chroma.2020.461477
- EU Reference Laboratories for Residues of Pesticides, SANTE/2019/12682, Analytical quality control and method validation procedures for pesticide residues analysis in food and feed (2020). https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf

