Stability Study of Mixed Neutral and Acidic Cannabinoid Standards
Abstract
This study examines the stability of working solutions prepared by combining neutral and acidic cannabinoid standards. Stability was assessed for 16 cannabinoids across 30 days for solutions prepared in commonly used diluents and stored under typical lab conditions.
Introduction
To ensure accurate reporting, it is essential that the stability of cannabinoid standards be studied. For example, if improperly manufactured or stored, tetrahydrocannabinolic acid (THCA) will convert to its methoxy counterpart, tetrahydrocannabinol (THC), giving invalid results for both analytes. To extend stability and simplify the preparation of working standards, Restek has formulated two certified reference materials (CRMs) comprised of 16 commonly analyzed cannabinoids. These standards are formulated in two high-concentration (1000 µg/mL) solutions: a cannabinoids acids 7 mix (cat.# 34144) and a cannabinoids neutrals 9 mix (cat.# 34132). Using these two CRMs instead of 16 single-compound standards saves labs time and money, and minimizes preparation errors. More important, because the acids and neutrals are formulated separately in appropriate diluents instead of as a single solution, long-term stability can be increased by preventing decarboxylation of the acids.
Stability studies on sealed ampuls of both the cannabinoid acids and neutrals CRMs have been performed by Restek under accelerated conditions for standard stability for up to four weeks. However, to assess the stability of working standards, the current study was conducted by combining the cannabinoid CRMs in commonly used diluents and storing the mixed solutions under typical lab conditions.
Experimental
The components of each cannabinoid CRM are given below. To measure the stability of a combined solution of Restek’s cannabinoids acids 7 standard (cat.# 34144) and cannabinoids neutrals 9 standard (cat.# 34132), duration, temperature, and diluent parameters (Figure 1) were selected to represent use in a typical production lab. Peak response (area) data was then collected and compared. Data was normalized to Day 0 results, and the acceptance criterion was defined as ±5% of the original response on Day 0.
Cannabinoids Acids 7 Standard (cat.# 34144)
1000 µg/mL, acetonitrile with 1% DIPEA and 0.05% ascorbic acid
- Cannabichromenic acid (CBCA) (185505-15-1)
- Cannabidiolic acid (CBDA) (1244-58-2)
- Cannabidivarinic acid (CBDVA) (31932-13-5)
- Cannabigerolic acid (CBGA) (25555-57-1)
- Cannabinolic acid (CBNA) (2808-39-1)
- Tetrahydrocannabinolic acid (THCA-A) (23978-85-0)
- Tetrahydrocannabivarinic acid (THCVA) (39986-26-0)
Cannabinoids Neutrals 9 Standard (cat.# 34132)
1000 µg/mL in purge-and-trap methanol
- Cannabichromene (CBC) (20675-51-8)
- Cannabicyclol (CBL) (21366-63-2)
- Cannabidiol (CBD) (13956-29-1)
- Cannabidivarin (CBDV) (24274-48-4)
- Cannabigerol (CBG) (25654-31-3)
- Cannabinol (CBN) (521-35-7)
- d8-Tetrahydrocannabinol (d8-THC) (5957-75-5)
- d9-Tetrahydrocannabinol (d9-THC) (1972-08-3)
- Tetrahydrocannabivarin (THCV) (31262-37-0)
Figure 1: Experimental Parameters for Testing the Stability of Prepared Cannabinoid Standards (50 ppm Mixtures of Acids and Neutrals)
Standard Solution Preparation
Prior to dilution, storage and handling of both cannabinoid CRMs followed their certificates of analysis (CoA), including the recommended storage condition of -20 ⁰C and sonication prior to use. Using aliquots from the same ampuls for each CRM, mixed standards were prepared at 50 ppm in each diluent to a final volume of 1 mL following the procedure in Figure 2. Each working standard was prepared in a 2 mL amber vial, capped, and then stored under one of the test conditions described in Figure 1.
Figure 2: Sample Preparation of 50 ppm Working Standards (Combined Cannabinoid Acids and Neutrals CRMs)
Analytical Conditions
Analytes were monitored using the following chromatographic conditions. On each test day, the prepared working standards were removed from their respective storage conditions, analyzed, recapped, and then returned to their storage conditions after analysis.
Column: Raptor ARC-18 2.7 µm 150 mm x 4.6 mm (cat. # 9314A65)
Injection volume: 5 µL
Mobile phase A: Water, 5 mM ammonium formate, 0.1% formic acid
Mobile phase B: Acetonitrile, 0.1% formic acid
Flow rate: 1.5 mL/min
Instrument: Shimadzu Nexera X2
Detector: Shimadzu DAD @ 228 nm
Temperature: 30 °C
Gradient: Isocratic, 75% B
Run time: 11 minutes
Results and Discussion
The example chromatogram in Figure 3 shows good peak shape and separation for all 16 neutral and acidic cannabinoids on a Raptor ARC-18 column. Each working standard was injected in triplicate, and the area values for each cannabinoid were averaged and normalized to the average Day 0 area values to produce the relative response results presented in Tables I and II and Figures 4-10. Overall, the data indicate that the cannabinoids in the mixed acids and neutrals working standards were stable across most diluent and storage condition combinations. Responses for all analytes were within the ±5% criteria, except for CBDV, CBG, and CBD in room temperature storage (25 ⁰C) on day 30. In addition, it was observed that the acetonitrile:water (75:25) diluent data showed an increasing response, which suggests evaporation of the diluent.
Figure 3: Representative Chromatogram for a 50-ppm Mixed Standard Blended from Acidic and Neutral Cannabinoid CRMs (Room Temperature, Day 30).
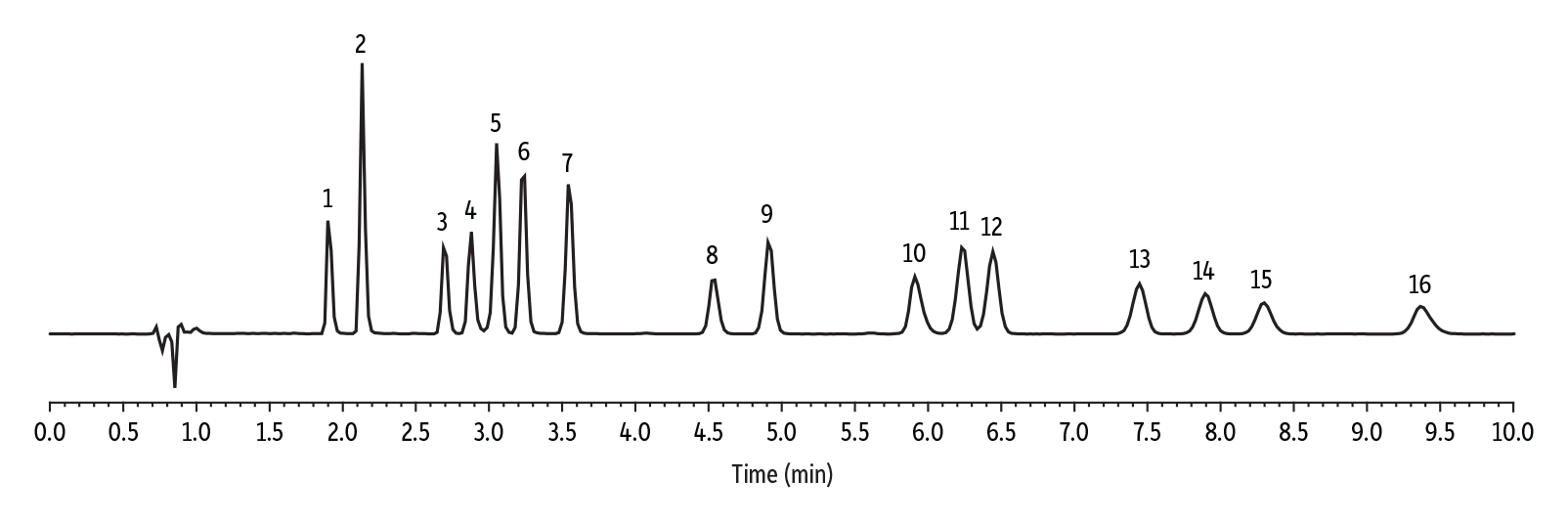
| Peaks | tR (min) | |
|---|---|---|
| 1. | Cannabidivarinic acid (CBDVA) | 1.91 |
| 2. | Cannabidivarin (CBDV) | 2.15 |
| 3. | Cannabidiolic acid (CBDA) | 2.69 |
| 4. | Cannabigerolic acid (CBGA) | 2.90 |
| 5. | Cannabigerol (CBG) | 3.05 |
| 6. | Cannabidiol (CBD) | 3.21 |
| 7. | Tetrahydrocannabivarin (THCV) | 3.58 |
| 8. | Tetrahydrocannabivarinic acid (THCVA) | 4.52 |
| Peaks | tR (min) | |
|---|---|---|
| 9. | Cannabinol (CBN) | 4.93 |
| 10. | Cannabinolic acid (CBNA) | 5.91 |
| 11. | Δ9-Tetrahydrocannabinol (Δ9-THC) | 6.23 |
| 12. | Δ8- Tetrahydrocannabinol (Δ8-THC) | 6.45 |
| 13. | Cannabicyclol (CBL) | 7.45 |
| 14. | Cannabichromene (CBC) | 7.90 |
| 15. | δ-9-Tetrahydrocannabinolic acid-A (THCA-A) | 8.30 |
| 16. | Cannabichromenic acid (CBCA) | 9.37 |
| Column | Raptor ARC-18 (cat.# 9314A65) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 150 mm x 4.6 mm ID | ||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||
| Pore Size: | 90 Å | ||||||||||||
| Temp.: | 30 °C | ||||||||||||
| Standard/Sample | Cannabinoids acids 7 standard, 1000 µg/mL, acetonitrile with 1% DIPEA and 0.05% ascorbic acid (cat.# 34144) | ||||||||||||
| Cannabinoids neutrals 9 standard, 1000 µg/mL, P&T methanol (cat.# 34132) | |||||||||||||
| Diluent: | Acetonitrile | ||||||||||||
| Conc.: | 50 ppm | ||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||
| Mobile Phase | |||||||||||||
| A: | Water, 5 mM ammonium formate, 0.1% formic acid | ||||||||||||
| B: | Acetonitrile, 0.1% formic acid | ||||||||||||
|
| Detector | Shimadzu DAD @ 228 nm |
|---|---|
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | To prepare the working standards, 50 µL of the cannabinoids acids 7 standard (cat.# 34144); 50 µL of the cannabinoids neutrals 9 standard (cat.# 34132); and 900 µL of acetonitrile were aliquoted into 2 mL, screw-thread vials (cat.# 21143), capped with short-cap, screw vial closures (cat.# 24498), and stored at room temperature for 30 days. |
Table I: Stability of Neutral Cannabinoids in Mixed Working Standards Stored Under Test Conditions (% Response Relative to Day 0). Green Indicates Result Passes the ±5% Acceptance Criteria, Yellow Indicates a Borderline Result, Red Indicates a Failing Result.
| Storage Temperature | - 20 ⁰C |
0 ⁰C |
|||||||
| Diluent | Acetonitrile |
Methanol |
Acetonitrile |
||||||
| Day | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 |
| Cannabidivarin | 103 | 101 | 102 | 98 | 99 | 98 | 102 | 102 | 104 |
| Cannabigerol | 103 | 102 | 103 | 99 | 98 | 98 | 102 | 103 | 103 |
| Cannabidiol | 103 | 102 | 102 | 98 | 98 | 98 | 102 | 102 | 103 |
| Tetrahydrocannabivarin | 103 | 102 | 102 | 98 | 99 | 98 | 104 | 102 | 103 |
| Cannabinol | 103 | 102 | 102 | 98 | 98 | 97 | 103 | 102 | 103 |
| ∆9-Tetrahydrocannabinol | 104 | 103 | 102 | 98 | 98 | 97 | 101 | 101 | 101 |
| ∆8-Tetrahydrocannabinol | 103 | 101 | 102 | 98 | 99 | 98 | 103 | 102 | 104 |
| Cannabicyclol | 104 | 103 | 102 | 99 | 99 | 97 | 102 | 102 | 103 |
| Cannabichromene | 104 | 102 | 102 | 98 | 98 | 97 | 102 | 102 | 103 |
| Storage Temperature | 10 ⁰C |
25 ⁰C |
||||||||||
| Diluent | Acetonitrile |
Methanol |
Acetonitrile: Water |
Acetonitrile |
||||||||
| Day | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 |
| Cannabidivarin | 99 | 100 | 100 | 98 | 98 | 97 | 102 | 102 | 102 | 101 | 98 | 88 |
| Cannabigerol | 99 | 102 | 101 | 98 | 99 | 99 | 102 | 103 | 103 | 99 | 95 | 87 |
| Cannabidiol | 99 | 101 | 101 | 97 | 98 | 98 | 102 | 103 | 103 | 102 | 99 | 93 |
| Tetrahydrocannabivarin | 99 | 101 | 101 | 97 | 98 | 98 | 102 | 103 | 104 | 103 | 102 | 103 |
| Cannabinol | 99 | 102 | 102 | 96 | 98 | 98 | 103 | 103 | 104 | 103 | 102 | 104 |
| ∆9-Tetrahydrocannabinol | 99 | 102 | 102 | 96 | 98 | 98 | 102 | 103 | 104 | 103 | 102 | 103 |
| ∆8-Tetrahydrocannabinol | 99 | 101 | 101 | 97 | 98 | 98 | 103 | 103 | 104 | 104 | 102 | 105 |
| Cannabicyclol | 99 | 102 | 102 | 96 | 98 | 98 | 102 | 103 | 104 | 104 | 103 | 104 |
| Cannabichromene | 99 | 102 | 102 | 96 | 98 | 98 | 102 | 103 | 104 | 104 | 103 | 105 |
Table II: Stability of Acidic Cannabinoids in Mixed Working Standards Stored Under Test Conditions (% Response Relative to Day 0). Green Indicates Result Passes the ±5% Acceptance Criteria, Yellow Indicates a Borderline Result, Red Indicates a Failing Result
| Storage Temperature | - 20 ⁰C | 0 ⁰C | |||||||
| Diluent | Acetonitrile | Methanol | Acetonitrile | ||||||
| Day | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 |
| Cannabidivarinic acid | 102 | 100 | 100 | 98 | 99 | 97 | 100 | 100 | 101 |
| Cannabidiolic acid | 102 | 100 | 100 | 98 | 98 | 97 | 101 | 101 | 101 |
| Cannabigerolic acid | 102 | 100 | 100 | 98 | 97 | 97 | 100 | 100 | 101 |
| Tetrahydrocannabivarinic acid | 102 | 100 | 101 | 98 | 98 | 97 | 100 | 100 | 101 |
| Cannabinolic acid | 101 | 101 | 101 | 98 | 98 | 97 | 100 | 101 | 101 |
| Tetrahydrocannabinolic acid | 102 | 104 | 100 | 98 | 98 | 97 | 100 | 100 | 101 |
| Cannabichromenic acid | 102 | 102 | 101 | 98 | 98 | 97 | 101 | 101 | 101 |
| Storage Temperature | 10 ⁰C | 25 ⁰C | ||||||||||
| Diluent | Acetonitrile | Methanol | Acetonitrile: Water |
Acetonitrile | ||||||||
| Day | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 | 10 | 15 | 30 |
| Cannabidivarinic acid | 97 | 99 | 99 | 96 | 98 | 97 | 99 | 100 | 101 | 101 | 99 | 95 |
| Cannabidiolic acid | 98 | 100 | 100 | 96 | 98 | 97 | 101 | 102 | 102 | 101 | 99 | 96 |
| Cannabigerolic acid | 98 | 99 | 99 | 96 | 97 | 97 | 101 | 102 | 103 | 101 | 98 | 95 |
| Tetrahydrocannabivarinic acid | 98 | 99 | 100 | 96 | 97 | 98 | 100 | 101 | 102 | 101 | 99 | 99 |
| Cannabinolic acid | 99 | 100 | 101 | 96 | 97 | 98 | 100 | 102 | 103 | 102 | 101 | 104 |
| Tetrahydrocannabinolic acid | 99 | 100 | 100 | 96 | 98 | 97 | 100 | 101 | 102 | 101 | 99 | 100 |
| Cannabichromenic acid | 100 | 101 | 101 | 97 | 98 | 98 | 101 | 102 | 103 | 103 | 101 | 103 |
Figure 4: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Acetonitrile, Stored at -20 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 5: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Acetonitrile, Stored at 0 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 6: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Acetonitrile, Stored at 10 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 7: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Acetonitrile, Stored at 25 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 8: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Methanol, Stored at 10 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 9: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Methanol, Stored at -20 ⁰C, and Analyzed on Days 0, 10, 15, and 30
Figure 10: Percent Response Results Relative to Day 0 for 16 Cannabinoids at 50 ppm in a Mixed Standard Prepared in Acetonitrile:Water (75:25); Stored at 10 ⁰C; and Analyzed on Days 0, 10, 15, and 30
Conclusion
A certificate of analysis for a CRM gives the expiration date of a properly stored, unopened ampul. Once opened, the solutions should be transferred to an appropriate vial and stored under recommended conditions between use. Like all analytes, cannabinoid stability is affected by the environmental and handling conditions to which the CRM and working standards are exposed. While Restek has completed stability studies on both sealed CRM ampuls and mixed working standard containing both acidic and neutral cannabinoids, it is strongly advised that labs conduct their own stability studies and implement SOPs to address the use and handling of CRMs and the preparation of working standards. During the current study, the cannabinoids were generally stable across the test conditions; however, for the most accurate results we recommend, especially when combining multicomponent ampuls, that standards be prepared fresh daily to prevent degradation. In addition, having technicians who prepare samples also prepare their own QC samples and calibration curves is a best practice because it can reduce variation. More information about Restek reference standard manufacturing and testing can be found at https://www.restek.com/articles/restek-reference-standards.

