Tech Tip: Column Conditioning Ensures Consistent EtG/EtS Results
Typical methods for analyzing EtG and EtS in human urine have several limitations: poor retention and resolution of EtG and EtS from matrix components, long run times that limit sample throughput, and short column lifetimes. Restek’s chemists have developed a simple, dilute-and-shoot LC-MS/MS method for EtG/EtS analysis using the Raptor EtG/EtS column, which provides consistent separations in a fast, four-minute analysis time.
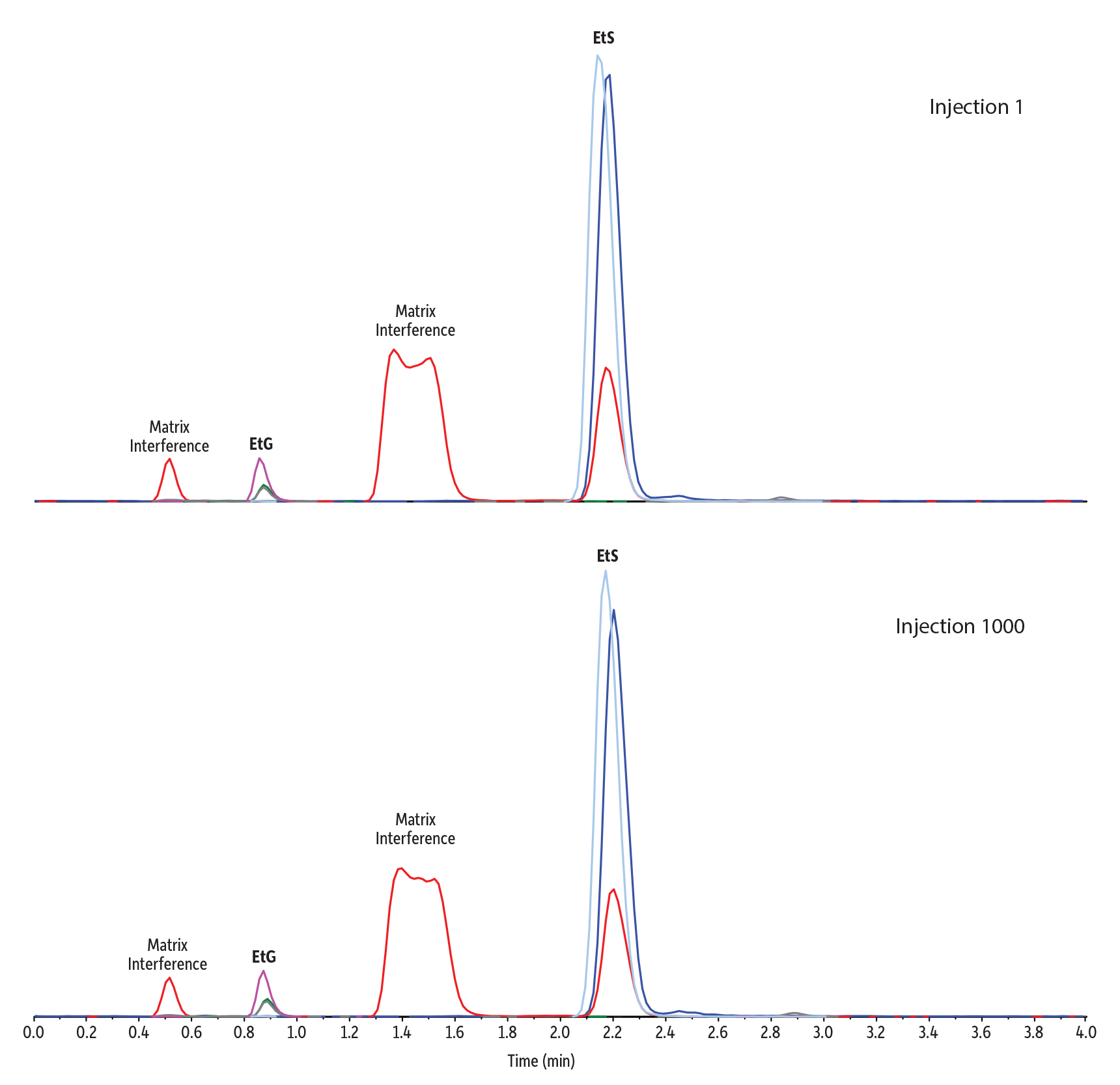
The Raptor EtG/EtS column features a novel stationary phase developed specifically for this critical application. This method was developed and stringently tested on the Raptor EtG/EtS column because it provides the retention needed to consistently elute the target analytes well away from matrix interferences and is rugged and long-lasting to satisfy the demands of high-throughput labs. As with all new columns, it is good practice to run a number of conditioning or equilibration injections in order to ensure good response, peak shape, and retention time consistency. As part of method development, we determined that running 30 matrix injections through the full gradient program on a new column ensured highly consistent performance during subsequent sample analysis (Figure 1).
By properly conditioning new columns and employing Restek’s EtG/EtS method for sample prep and analysis, high-throughput labs testing human urine samples for these alcohol consumption biomarkers can ensure accurate, consistent results with fewer column changes. Full method parameters, verification data, and analyses of patient samples are provided here.
Figure 1: Highly consistent EtG/EtS results are obtained using a preconditioned Raptor EtG/EtS column and the method parameters shown here. Matrix interferences are still well resolved even after 1,000 sample injections.
| Peaks | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|
| 1. | Ethyl-β-D-glucuronide-d5 | 225.9 | 84.9 | - |
| 2. | Ethyl-β-D-glucuronide | 220.8 | 84.9 | 74.8 |
| 3. | Ethyl sulfate-d5 | 129.7 | 97.7 | - |
| 4. | Ethyl sulfate | 124.7 | 96.8 | 79.7 |
| Column | Raptor EtG/EtS (cat.# 9325A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | UltraShield UHPLC precolumn filter, 0.2 µm frit (cat.# 25809) | ||||||||||||||||||||
| Temp.: | 35 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 0.1% Formic acid in water | ||||||||||||||||||||
| Conc.: | A 500 ng/mL QC sample was prepared in urine. 50 µL of the sample was diluted with 950 µL of a working internal standard (25 ng/mL EtS-d5/100 ng/mL EtG-d5 in 0.1% formic acid in water). The sample was vortexed at 3500 rpm for 10 seconds to mix. The sample was then centrifuged at 3000 rpm for 5 minutes at 10 °C. The autosampler needle was adjusted to inject from the supernatant. | ||||||||||||||||||||
| Inj. Vol.: | 10 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | HPLC |
| Notes | Reference Standards Ethyl-β-D-glucuronide (cat.# 34101) Ethyl-β-D-glucuronide-d5 (cat.# 34102) Ethyl sulfate sodium salt (cat.# 34103) Ethyl sulfate-d5 sodium salt (cat.# 34104) |

