A Nitrosamines CRM and Method Development Utilizing Restek’s Pro EZLC
26 Mar 2024We can be exposed to nitrosamines through our daily routines. They are largely unavoidable because they can exist in air, water, food, and pharmaceuticals to name a few routes of exposure. There is data that supports a link between elevated nitrosamine exposure and cancer. Therefore, action must be taken to limit our exposure to these harmful compounds. Focusing on pharmaceuticals, regulatory agencies, such as the FDA and EMA, have issued guidance drawn from internal research ICH recommendations regarding drug manufacturing and acceptable intake (AI) limits.
Simply put, what makes a compound a nitrosamine is the presence of the nitrosoamino group shown here:
There are countless compounds we can call nitrosamines. They can form from amines (secondary, tertiary, and quaternary) in the presence of nitrite salts under acidic conditions. These types of amines are also referred to as nitrosamine precursors. See below an example of nitrosamine formation from the FDA’s Nitrosamine Guidelines[1]:
Nitrosamines can also form in the presence of an active pharmaceutical ingredient (API) during the drug manufacturing process as byproducts of the intended synthesis. These byproducts are called Nitroso-Drug Substance Related Impurities (NDSRI’s).
A recent example of the need to monitor for all possible formations of nitrosamines is from July 2021. An API (Varenicline tartate, Chantix) that is prescribed as a smoking cessation aid had specific lots recalled due to this inadvertent creation of an NDSRI, N-nitroso-varenicline[4]. The FDA went so far as to publish a method using LC-ESI-HRMS titled Liquid Chromatography-High Resolution Mass Spectrometry (LC-ESI-HRMS) Method for the Determination of Varenicline Nitroso-Drug Substance Related Impurity (NDSRI) in Chantix™ Drug Product and Varenicline Drug Substance[5]. The complication with NDSRI’s is that there is not always a reference standard available specific to the parent compound, so other means need to be utilized to monitor NDSRI’s, including LC/QTOF. Beyond detection, synthesis of a reference standard would be useful for quantitation and regular quality testing by LC/MS/MS.
The FDA guidelines are found in the Control of Nitrosamine Impurities in Human Drugs[1]. This Guidance for Industry lists seven potential nitrosamine impurities that can theoretically exist in API’s and drug products. It is because of this guidance that we at Restek sought to develop a reference material that would streamline the analysis of these 7 nitrosamines (P/N: 36025).
The seven theoretical nitrosamine impurities and structures are[1]:
*Please note the stereocenter around the secondary nitrogen.
Curious about chromatographic separation and overall workflow, I utilized Pro EZLC to see how they might resolve on our Raptor Biphenyl.
I started by selecting Nitrosamines as a compound class.
Then, I selected all the nitrosamines in this certified reference material and chose Raptor Biphenyl from the drop-down menu under Phase. Note that they are many other common nitrosamines present for exploration and method development.
Next, I clicked Generate Model.
I tabbed over to Conditions to play with column dimensions, column temperature and flow rates. Keep in mind the Back Pressure calculation to make sure you aren’t exceeding your max pressure for your instrumentation.
There are many directions you can take in method development. Utilize the tools in Pro EZLC to achieve your goals and find balance for what is most important to you and your lab. These are the goals I had in mind while utilizing EZLC:
- Compound Retention: I didn’t want any compound eluting before 1 minute.
- Reproducibility: I wanted to add enough volume for at least 5 column rinses for re-equilibration.
- Green Thumb: I wanted to lower my flow rates as much as possible while still maintaining back pressure for sharp resolution.
- Fast LC: Toggling temperatures to maintain backpressure while balancing elution times.
- Accessibility: I wanted to keep my max pressure under 6000psi to show separations without UHPLC.
Here are the conditions that I landed on for my goals with this assay:
Here is the chromatogram I would expect following the conditions and column selection using this reference standard:
Here are the calculated RT’s and MS conditions:
Time to test our Pro EZLC software against a real experiment. We plumbed the Raptor Biphenyl (cat.# 9309A12) and equilibrated the conditions on a Shimadzu Nexera X2. The compounds were optimized on a SCIEX 4500 MS/MS. The ionization technique was APCI+. APCI is preferred for nitrosamines over ESI. It has been reported to provide better sensitivity and reproducibility.[2]
Here are the results:
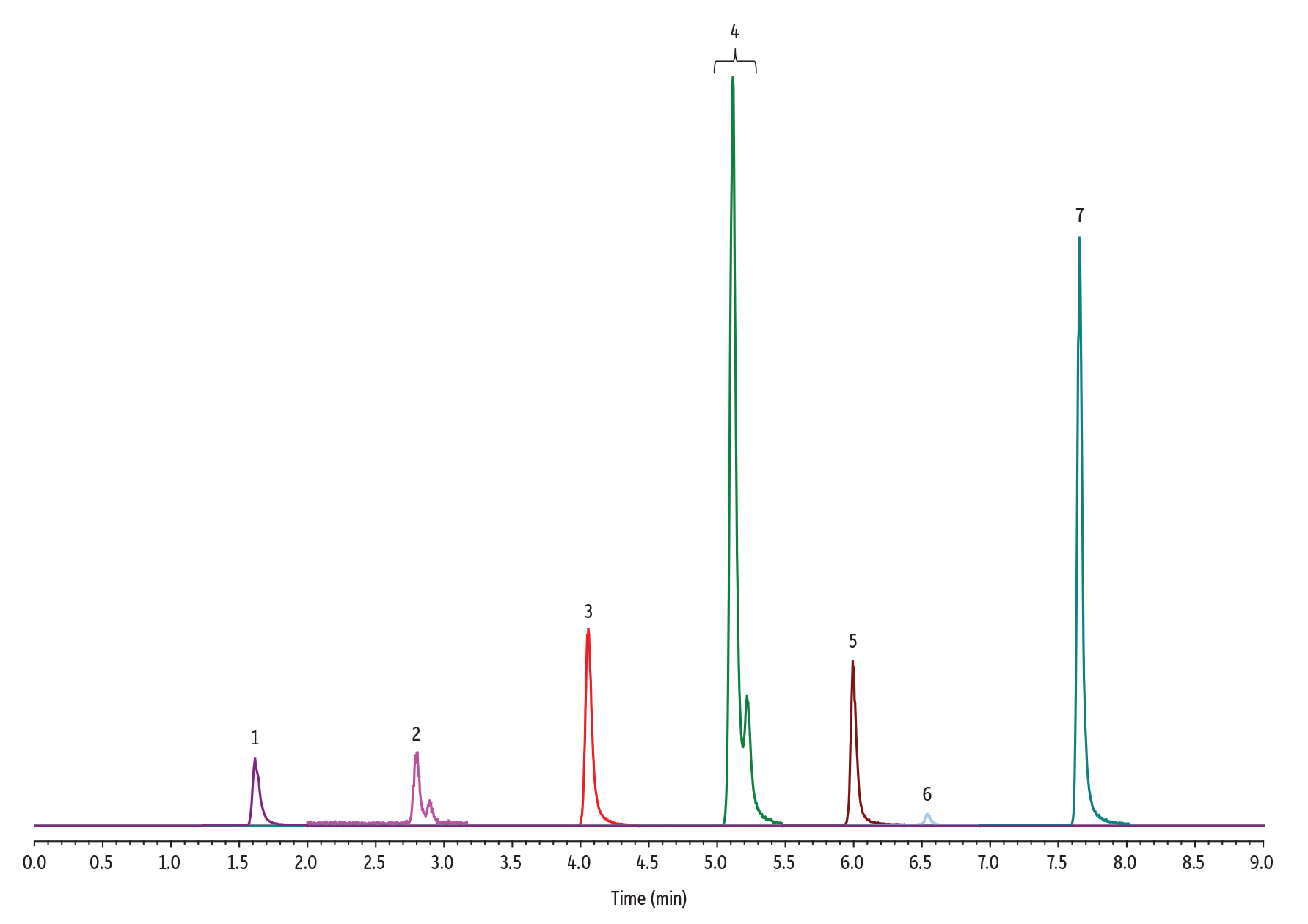
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | N-Nitrosodimethylamine (NDMA) | 1.62 | 75.1 | 58.0 |
| 2. | N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) | 2.79 | 147.0 | 117.1 |
| 3. | N-Nitrosodiethylamine (NDEA) | 4.05 | 103.1 | 75.0 |
| 4. | N-Nitrosoethylisopropylamine (NEIPA) | 5.10 | 117.1 | 75.0 |
| 5. | N-Nitrosodiisopropylamine (NDIPA) | 5.99 | 131.2 | 43.1 |
| 6. | N-Nitrosomethylphenylamine (NMPA) | 6.55 | 137.0 | 65.9 |
| 7. | N-Nitrosodibutylamine (NDBA) | 7.64 | 159.1 | 57.0 |
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | Nitrosamines 7 standard (cat.# 36025) | ||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||
| |||||||||||||||||||||
| Max Pressure: | 340 bar |
| Detector | SCIEX 4500 MS/MS |
|---|---|
| Ion Source: | APCI |
| Ion Mode: | APCI+ |
| Mode: | Scheduled MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | A 100 ng/mL stock was prepared by adding 1 µL of nitrosamines 7 standard to 999 µL of water in 2 mL, screw-thread amber vials (cat.# 21143) and capped with 9 mm, short-cap, screw-vial closures (cat.# 24498). |
Comparing the two tables, we see that there is very little difference between retention times in the Pro EZLC tool and the actual results using identical conditions.
| Compound | Pro EZLC | Actual | Percent Difference |
| NDMA | 1.56 | 1.62 | 3.84 |
| NMBA | 2.79 | 2.79 | 0 |
| NDEA | 4.06 | 4.05 | 0.24 |
| NEIPA | 5.1 | 5.1 | 0 |
| NDIPA | 5.97 | 5.99 | 0.33 |
| NMPA | 6.49 | 6.55 | 0.92 |
| NBDA | 7.61 | 7.64 | 0.39 |
When I first analyzed this chromatogram, it was concerning to me that there is some peak splitting. Investigating this further, we can expect a mixture of chiral compounds, and thus peak doublets, from NMBA, NEIPA and NMPA[2]. These compounds have stereocenters around their secondary nitrogen. It is impossible to isolate these nitrogenic stereocenters due to their rapid interconversion of their enantiomers.
This poses a great question: Is the additional selectivity of our Raptor biphenyl the ideal column for this analysis?
It makes the most sense to avoid this separation of enantiomers. If nitrosamines are present in an unknown, we want to account for the total nitrosamine. This means we would need to integrate both peaks in all samples, calibrators, and controls. This creates potential possibility of error because it introduces a factor of interpretation alongside co-eluting peaks that can adversely affect accuracy and precision. It also leads to less sensitivity, given that these compounds are not stacked on top of each other contributing to a higher S/N. Referencing USP General Chapter <1469> Nitrosamine Impurities, Procedure 3, USP recommends an L1 column[3]. This is more commonly known as a C18 column. Going back to the drawing board, and running the exact same assay using our Raptor C18 we observe the following chromatogram:
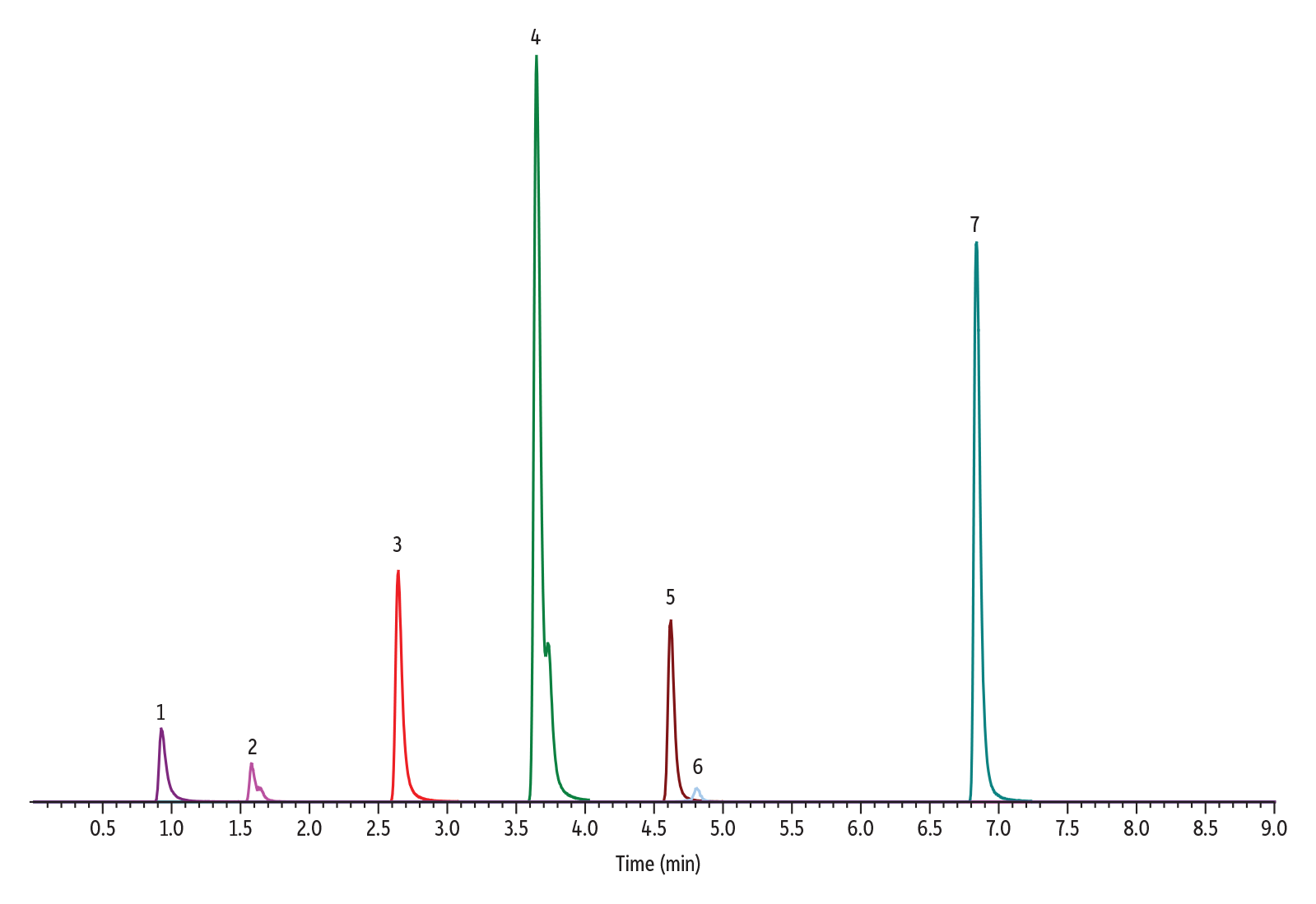
| Peaks | tR (min) | Precursor Ion | Product Ion | |
|---|---|---|---|---|
| 1. | N-Nitrosodimethylamine (NDMA) | 0.93 | 75.1 | 58.0 |
| 2. | N-Nitroso-N-methyl-4-aminobutyric acid (NMBA) | 1.58 | 147.0 | 117.1 |
| 3. | N-Nitrosodiethylamine (NDEA) | 2.65 | 103.1 | 75.0 |
| 4. | N-Nitrosoethylisopropylamine (NEIPA) | 3.66 | 117.1 | 75.0 |
| 5. | N-Nitrosodiisopropylamine (NDIPA) | 4.61 | 131.2 | 43.1 |
| 6. | N-Nitrosomethylphenylamine (NMPA) | 4.82 | 137.0 | 65.9 |
| 7. | N-Nitrosodibutylamine (NDBA) | 6.84 | 159.1 | 57.0 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | Nitrosamines 7 Standard (cat.# 36025) | ||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 5 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water, 0.1% formic acid | ||||||||||||||||||||
| B: | Methanol, 0.1% formic acid | ||||||||||||||||||||
| |||||||||||||||||||||
| Max Pressure: | 340 bar |
| Detector | SCIEX 4500 MS/MS |
|---|---|
| Ion Source: | APCI |
| Ion Mode: | APCI+ |
| Mode: | Scheduled MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | A 100 ng/mL stock was prepared by adding 1 µL of Nitrosamines 7 Standard to 999 µL of water in 2 mL, screw-thread amber vials (cat.# 21143) and capped with 9 mm, short-cap, screw-vial closures (cat.# 24498). |
As you can see, the C18 still has some peak splitting for the compounds that can form enantiomers. However, there is more overlap and enough resolution between all compounds. There is some co-elution between NDIPA and NMPA. However, these compounds are not isobars, and the MS can easily differentiate between the two.
Pro EZLC is a great tool for beginners to experts in LC/MS. It’s especially useful for new applications that you may not be familiar with. Be aware of the possibility of separating out racemic mixtures when using biphenyl.
If you find Pro EZLC helpful and utilize GC, be sure to check out Pro EZGC too.
References:
- https://www.fda.gov/media/141720/download#:~:text=Because%20nitrosamines%20are%20probable%20or,their%20APIs%20and%20drug%20products.
- https://pubmed.ncbi.nlm.nih.gov/35696119/
- https://www.usp.org/sites/default/files/usp/document/stakeholder-forum/pnp/highlights-of-1469-nitrosamine-impurities.pdf
- https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-and-press-announcements-nitrosamine-varenicline-chantix
- https://www.fda.gov/media/151470/download?attachment
