Molecular Sieve Packed Columns and Fixed (Permanent) Gas Analysis
2 Dec 2013For some reason, I find molecular sieve packed columns interesting. Maybe it’s because they have a niche as the go-to columns for fixed (permanent) gas analysis. Maybe it’s because they are old school technology that a new generation of analysts are always asking questions about. Maybe it’s because using them successfully is a function of skill, art, and some luck.
As I researched molecular sieve packed columns over the years, I realized that for something that has been around more than 50 years, there wasn’t a whole lot of published information about them (at least not that I could find). Application chromatograms were few and far between, and troubleshooting information seemed even rarer. As a result, I decided to write this post on molecular sieve packed columns to help our customers. This information came from books, the internet, coworkers, or was provided by our customers. If you have any additional or contradictory information, I would love to hear from you.
I have written two other posts on molecular sieve packed columns. One was titled “ShinCarbon columns – Installation / Conditioning / Helpful Hints”, and the other “Molecular Sieve 5A & 13X packed columns - Installation / Conditioning / Helpful Hints”. So what’s the difference between these columns? Let’s start with Molecular Sieve 5A and 13X packed columns.
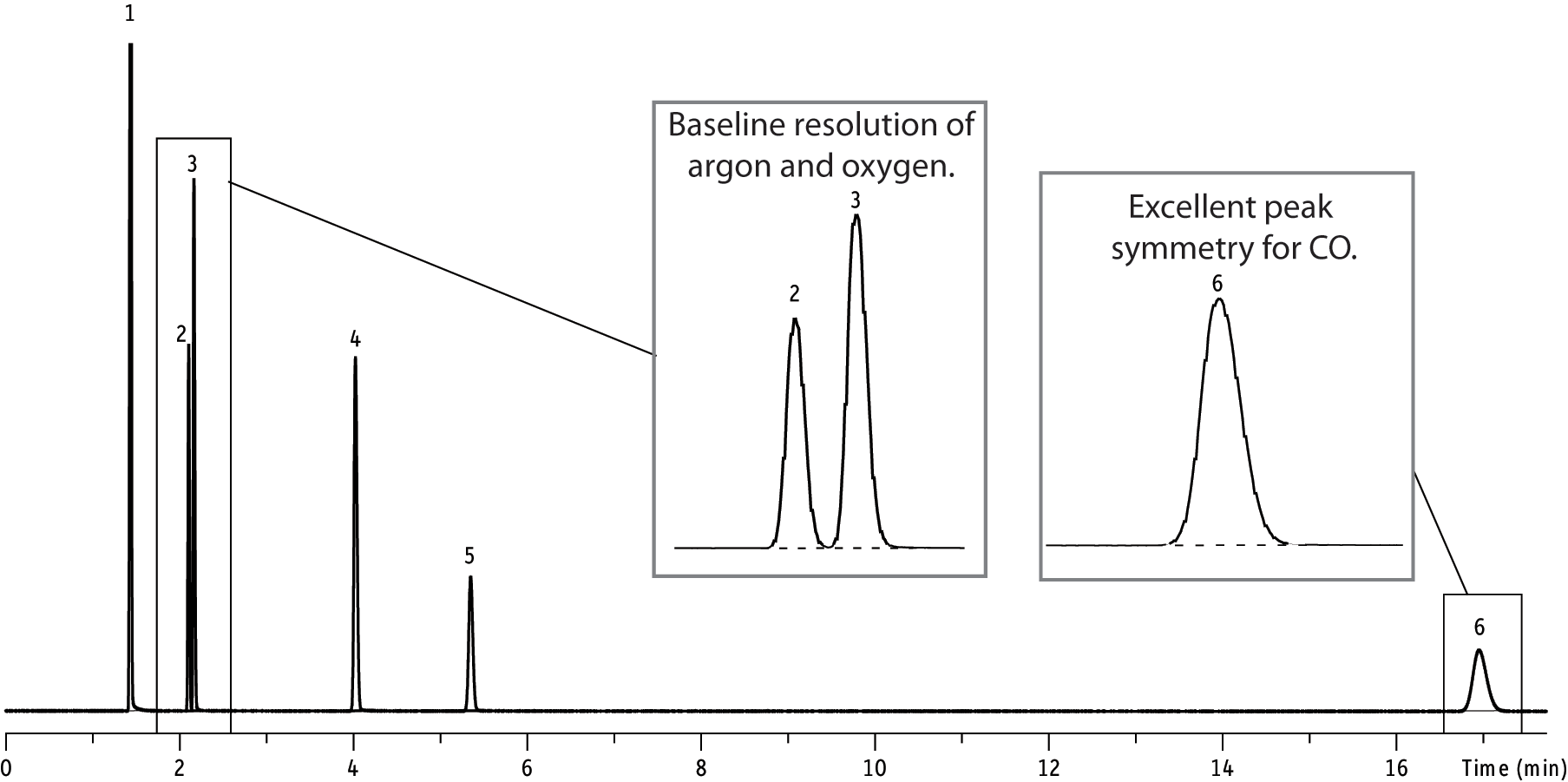
| Peaks | |
|---|---|
| 1. | Oxygen |
| 2. | Nitrogen |
| 3. | Methane |
| 4. | Carbon monoxide |
| Columns | Molesieve 5A 1 m, 2 mm ID (cat.# 80440-800) |
|---|---|
| and Molesieve 13X 2 m, 2 mm ID (cat.# 80439-800) | |
| Standard/Sample | 5-10% each component, balance helium |
| Injection | |
| Inj. Vol.: | 10 µL Direct |
| Inj. Temp.: | 150 °C |
| Oven | |
| Oven Temp.: | 50 °C |
| Carrier Gas | He, constant flow |
| Flow Rate: | 30 mL/min |
| Detector | TCD @ 200 °C |
|---|
Molecular Sieve 5A and 13X
Molecular Sieve 5A and 13X are referred to as zeolite molecular sieves. These crystalline materials are synthetically produced and have very uniform internal cavities. I am not going to discuss structural/composition details of Molecular Sieve type A materials verses Molecular Sieve type X materials, but rather focus on helping new analysts understand these packing and their applications a little better.
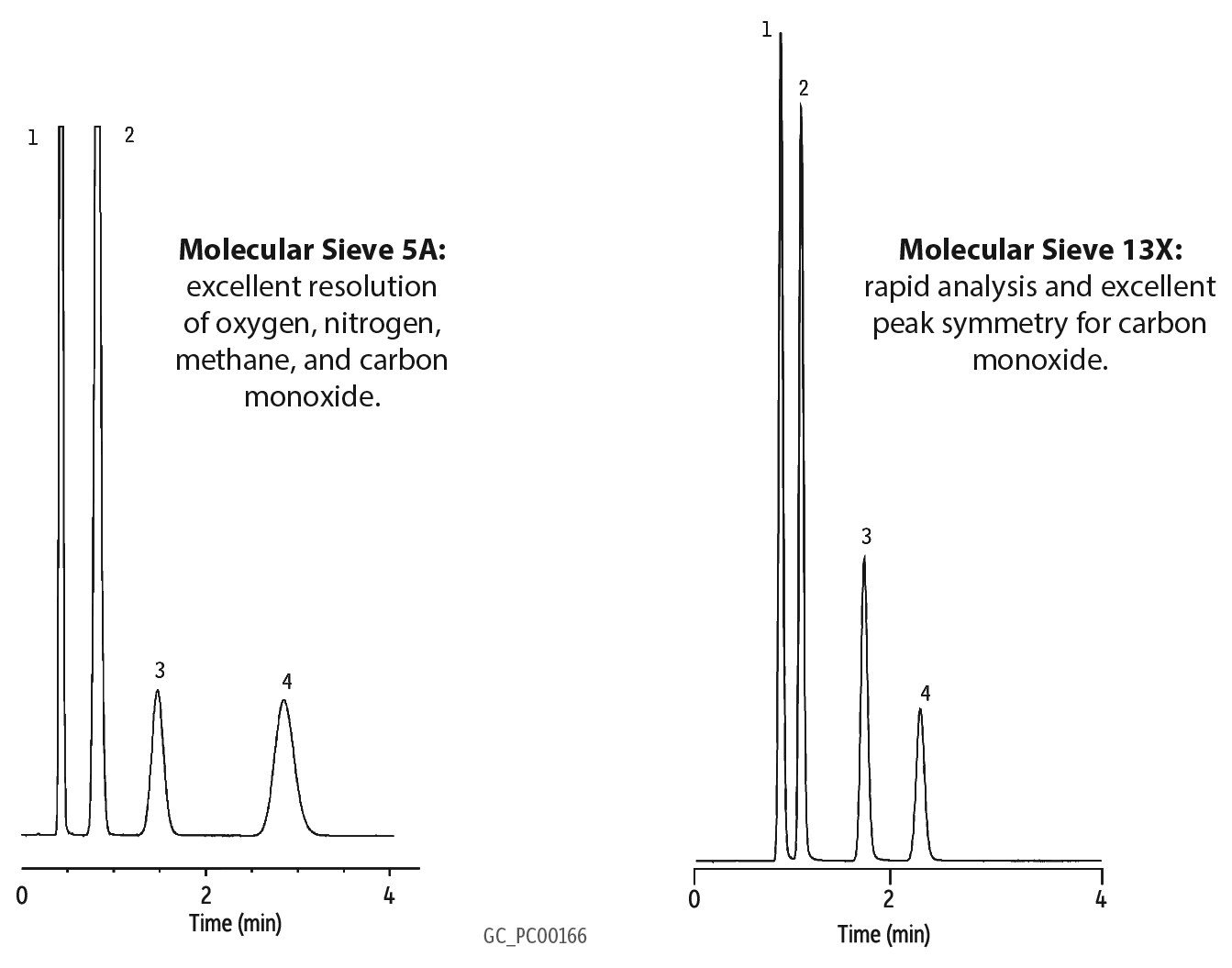
So what is the difference between 5A and 13X? Well, as I already mentioned, material structure is one difference. Pore size is another; a 5A has an effective pore size of 5 angstroms and a 13X has an effective pore size of 10 angstroms. In order for a compound to be able to pass through these pores, it must be smaller. For the compounds I plan to discuss (He, H2, Ar, O2, N2, CH4, and CO), each is able to pass through these pores.
So which compounds won’t pass through these pores? Well, I’m going to answer a different question and list several common gaseous compounds which should not be introduced onto these columns. These compounds include CO2, H2O, SO2, H2S, Cl2, HCl, NH3 (and other corrosive vapors), compounds larger than (and including) C2 (ethane) and/or compounds with boiling points greater than approximately 50C (please note: there are several high temperature hydrocarbon applications, but I will not discuss them here). Larger gaseous compounds may actually make it through the column, but many will have no retention because they cannot fit into the pores (like SF6 when using 5A). Other larger compounds may simply not make it through the column.
So what are some of the advantages of using these columns for gas analysis? The primary advantage is baseline separation between O2 and N2 with a relatively short column and at room temperature. Another is the separation of CO from air and CH4 (a difficult separation for any non-molecular sieve packed column).
So what are the disadvantages of these columns? The primary disadvantage is that these packings will trap CO2 and H2O, which is why these columns are typically used with a pre-column (stripper column) and valve switching. However, if CO2 and H2O do get trapped in these columns, the effects are reversible (see “Molecular Sieve 5A & 13X packed columns - Installation / Conditioning / Helpful Hints”).
Although zeolite molecular sieve packings are commonly used for argon/oxygen separation, a disadvantage of using a packed column is that a very long length (approximately 24ft from what I have read) is needed to separate oxygen from argon at room temperature. For (O2/Ar) separation, we usually recommend one of our Molecular Sieve 5A PLOT columns.
| Column | MXT-Msieve 5A, 30 m, 0.53 mm ID, 50 µm (cat.# 79723) |
|---|---|
| Standard/Sample | |
| Conc.: | 1% in hydrogen |
| Injection | split (split ratio 50:1) |
| Oven | |
| Oven Temp.: | 30 °C |
| Carrier Gas | H2 |
| Detector | μ-TCD |
|---|
Finally, one needs to be cautious when using any carrier gas other than nitrogen to dry/condition these columns. Because these materials contain surface metals that cannot be easily removed, it is believed that other carrier gases like hydrogen, argon and helium (at high temperatures) can remove the oxidation layer on the metal’s surface, which can lead to activity (especially for CO).
ShinCarbon ST
ShinCarbon ST is a synthetically manufactured carbon molecular sieve material with a very high surface area of approximately 1500m2/gm and a pore size of 10-12 angstroms. Its surface is very inert, and very non-polar. It is typically used for separation of fixed gases and light hydrocarbons, but also can be used for the analysis of % levels of SO2 (usually elutes after ethane) and ppm / % levels N2O (usually elutes after CO2 but before acetylene).
So how does a carbon molecular sieve column (ShinCarbon) compare to a zeolite molecular sieve (5A & 13X) column? Let’s start by looking where it doesn’t quite match-up with a 5A or 13X. First, it can be difficult to get baseline separation between O2 and N2 using a carbon molecular sieve column (customers report that a 10ft x 1/8” OD column is needed). In addition, it is even more difficult to separate O2/Ar (I have read that cryogenic cooling is a necessity). And just like the 5A & 13X, a ShinCarbon isn’t meant for H2S or any corrosive gases like Cl2, HCl, NH3.
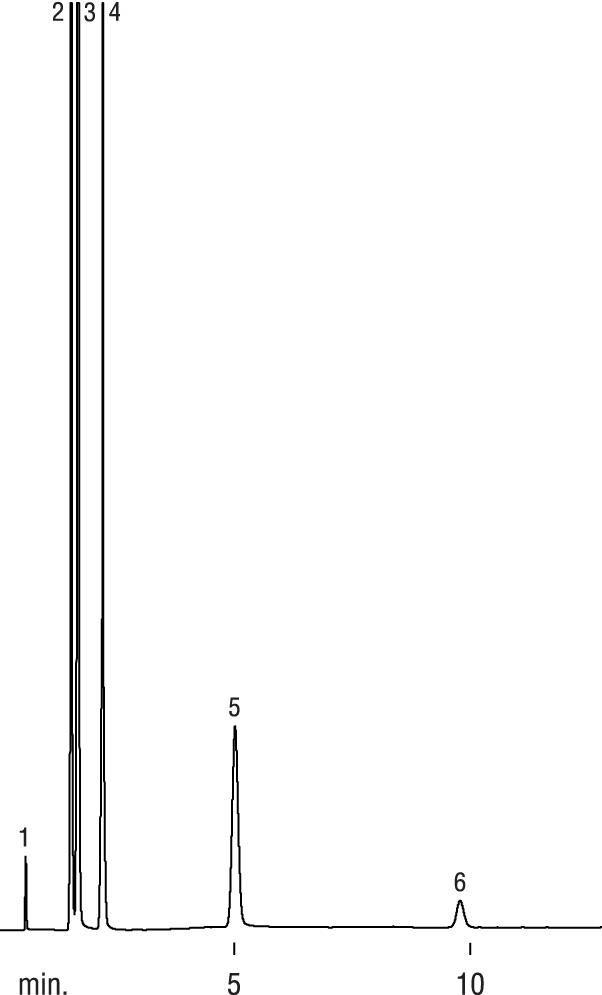
| Peaks | |
|---|---|
| 1. | Hydrogen |
| 2. | Oxygen |
| 3. | Nitrogen |
| 4. | Carbon monoxide |
| 5. | Methane |
| 6. | Carbon dioxide |
| Column | 100/120 mesh, ShinCarbon ST, 2 m, 1/16 in. OD, 1.0 mm ID (cat.# 19808) |
|---|---|
| Standard/Sample | Permanent gases mix, approx. 5 mol % each |
| Injection | |
| Inj. Vol.: | 5 µL packed on-column |
| Inj. Temp.: | 100 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 3 min) to 250 °C at 8 °C/min (hold 10 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 10 mL/min |
| Detector | HID @ 250 °C |
|---|
So why would you use a ShinCarbon? First, unlike zeolite molecular sieves, CO2, SO2 and H2O will not get trapped in the pores (Notes: it will be difficult to quantitate H2O because the peak will tail; there are better column choices for SO2). Plus, you can easily chromatograph larger compounds (like straight-chain hydrocarbons up through and including pentane). However, some unsaturated hydrocarbons (in particular, C3+) may show a lower response than expected, especially at higher temperatures. Hexanes will elute but tail.
If H2O does condense inside the column, 30 minutes at 250⁰C will dry a ShinCarbon (unlike the 5A & 13X which needs 300⁰C for 3 hours), and using a carrier gas other than nitrogen does not seem to add surface activity. Personally I do not suggest leaving it at higher temperatures (>250⁰C) for more than 3-hours (and certainly not overnight) since some customers have reported that the packing could develop surface activity under these conditions.
To determine the complete catalog number to purchase a 1/8" OD Shincarbon column (including the required suffix number), please review this blog post "Things to Consider Before Ordering a Packed Column".
I hope that you have found this information useful. As I mentioned, if you have any additional information, or you had an experience which is contradictory to something here, please let me know. Thanks for reading.