PFAS - EPA 1633 and Bile Acids
3 Apr 2023If you’re here, it’s likely you’ve heard about or read the EPA draft titled “Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS.” In it, the interference from bile acids to PFOS is outlined along with guidelines to follow for the analysis of 41 PFAS compounds. This potential interference from the bile acids with PFOS is affected by the organic modifier used in the analytical method. The document states that when using acetonitrile as the organic modifier, the bile acid taurodeoxycholic acid (TDCA) needs to be monitored and resolved with at least 1 minute or more between the bile acid and PFOS. If using any other organic modifier, such as methanol, it is necessary to also include taurochenodeoxycholic acid (TCDCA) and tauroursodeoxycholic acid (TUDCA) and achieve 1 minute or greater retention between PFOS and all three bile acids. In addition to the retention time requirement, the bile acids may also not coelute with any of the PFAS analytes. A method was developed to meet these guidelines using acetonitrile.
EPA 1633 PFAS on Force C18 by LC-MS/MS using Acetonitrile
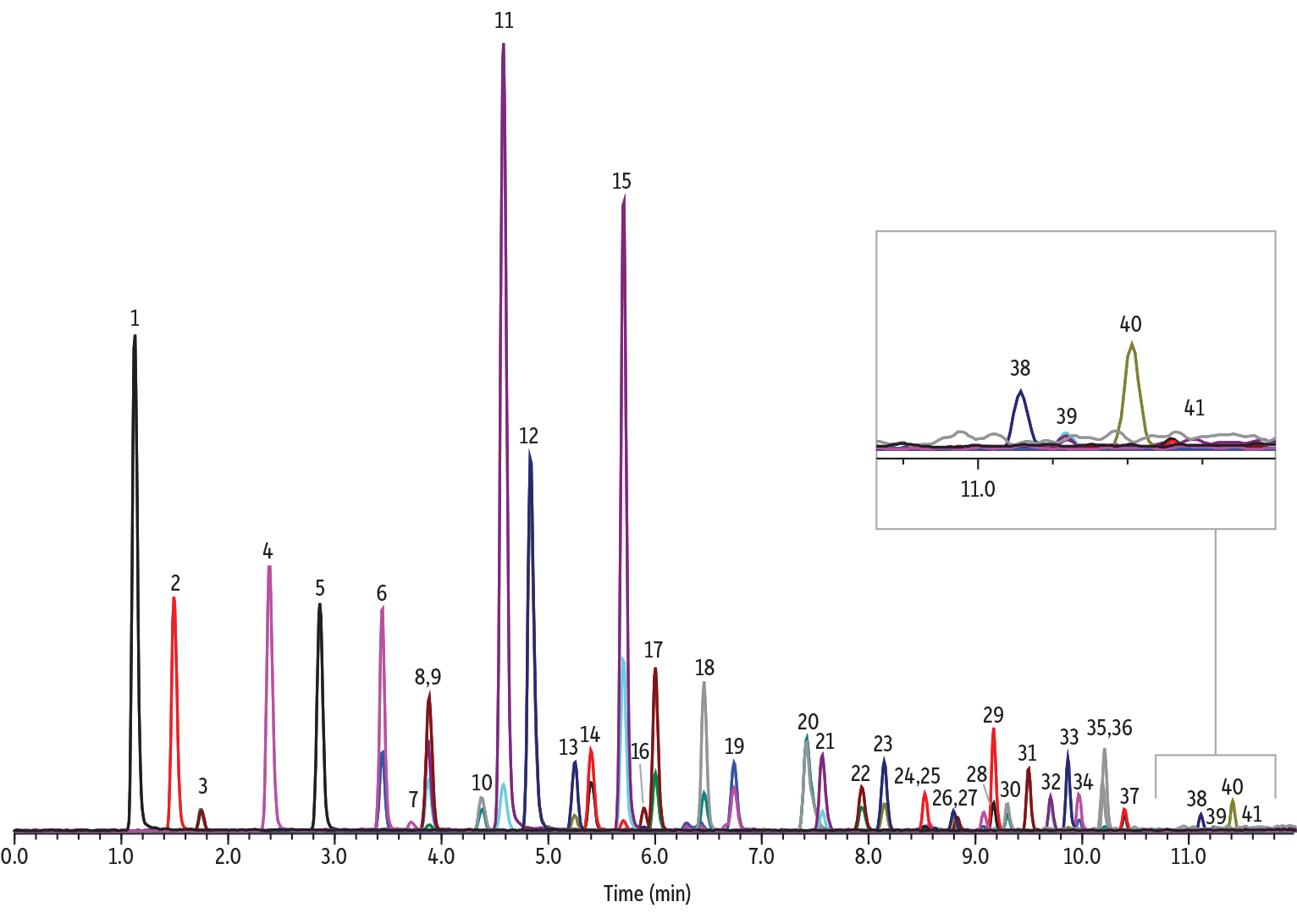
| Peaks | tR (min) | Conc. (ng/mL) | Precursor 1 | Product 1 | Product 2 | Precursor 2 | Product 1 | |
|---|---|---|---|---|---|---|---|---|
| 1. | Perfluorobutanoic acid (PFBA) | 1.13 | 200 | 212.8 | 169.0 | - | - | - |
| 2. | Perfluoro-3-methoxypropanoic acid (PFMPA) | 1.49 | 100 | 229.0 | 85.0 | - | - | - |
| 3. | 3-Perfluoropropyl propanoic acid (3:3FTCA) | 1.75 | 200 | 241.0 | 177.0 | 117.0 | - | - |
| 4. | Perfluoropentanoic acid (PFPeA) | 2.39 | 100 | 263.0 | 219.0 | 68.9 | - | - |
| 5. | Perfluoro-4-methoxybutanoic acid (PFMBA) | 2.86 | 100 | 279.0 | 85.0 | - | - | - |
| 6. | 1H,1H, 2H, 2H-Perfluorohexane sulfonic acid (4:2 FTS) | 3.44 | 200 | 327.0 | 307.0 | 81.0 | - | - |
| 7. | Nonafluoro-3,6-dioxaheptanoic acid (NFDHA) | 3.71 | 100 | 294.9 | 201.1 | 85.0 | - | - |
| 8. | Perfluorobutanesulfonic acid (PFBS) | 3.87 | 50 | 299.0 | 80.0 | 99.0 | - | - |
| 9. | Perfluorohexanoic acid (PFHxA) | 3.88 | 50 | 313.0 | 269.0 | 119.0 | - | - |
| 10. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 4.37 | 100 | 285.0 | 169.0 | 185.0 | - | - |
| 11. | Perfluoro(2-ethoxyethane)sulfonic acid (PFEESA) | 4.58 | 100 | 315.0 | 135.0 | 83.0 | - | - |
| 12. | 2H,2H,3H,3H-Perfluorooctanoic acid (5:3FTCA) | 4.83 | 20 | 341.0 | 237.0 | 217.0 | - | - |
| 13. | Perfluoroheptanoic acid (PFHpA) | 5.25 | 50 | 363.1 | 319.0 | 169.0 | - | - |
| 14. | Perfluoropentansulfonic acid (PFPeS) | 5.40 | 50 | 349.0 | 80.0 | 99.0 | - | - |
| 15. | 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 5.70 | 100 | 377.0 | 251.0 | 84.8 | - | - |
| 16. | Taurodeoxycholic acid (TDCA) | 5.90 | 100 | 498.4 | 80.0 | - | - | - |
| 17. | 1H,1H, 2H, 2H-Perfluorooctane sulfonic acid (6:2 FTS) | 6.00 | 200 | 427.0 | 407.0 | 81.0 | - | - |
| 18. | Perfluorooctanoic acid (PFOA) | 6.46 | 50 | 413.0 | 369.0 | 169.0 | - | - |
| 19. | Perfluorohexanesulfonic acid (PFHxS) | 6.74 | 50 | 399.0 | 80.0 | 99.0 | - | - |
| 20. | 3-Perfluoroheptyl propanoic acid (7:3FTCA) | 7.42 | 20 | 441.0 | 317.0 | 337.0 | - | - |
| 21. | Perfluorononanoic acid (PFNA) | 7.57 | 50 | 463.0 | 419.0 | 219.0 | - | - |
| 22. | Perfluoroheptanesulfonic acid (PFHpS) | 7.94 | 50 | 449.0 | 80.0 | 99.0 | - | - |
| 23. | 1H,1H, 2H, 2H-Perfluorodecane sulfonic acid (8:2 FTS) | 8.15 | 200 | 527.0 | 507.0 | 81.0 | - | - |
| 24. | Perfluorodecanoic acid (PFDA) | 8.53 | 50 | 513.0 | 469.0 | 219.0 | - | - |
| 25. | N-methyl perfluorooctanesulfonamidoacetic acid (NMeFOSAA) | 8.57 | 50 | 570.0 | 419.0 | 483.0 | - | - |
| 26. | Perfluorooctanesulfonic acid (PFOS) | 8.80 | 50 | 499.0 | 80.0 | 99.0 | - | - |
| 27. | N-ethyl perfluorooctanesulfonamidoacetic acid (NEtFOSAA) | 8.83 | 50 | 584.0 | 419.0 | 526.0 | - | - |
| 28. | Perfluoroundecanoic acid (PFUnA) | 9.08 | 50 | 563.0 | 519.0 | 269.0 | - | - |
| 29. | 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS) | 9.17 | 100 | 531.0 | 351.0 | - | 533.0 | 353.0 |
| 30. | Perfluorononanesulfonic acid (PFNS) | 9.30 | 50 | 549.0 | 80.0 | 99.0 | - | - |
| 31. | Perfluorododecanoic acid (PFDoA) | 9.50 | 50 | 612.7 | 568.8 | 319.0 | - | - |
| 32. | Perfluorodecanesulfonic acid (PFDS) | 9.70 | 50 | 598.6 | 80.0 | 99.0 | - | - |
| 33. | Perfluorotridecanoic acid (PFTrDA) | 9.87 | 50 | 662.8 | 618.8 | 169.0 | - | - |
| 34. | 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 9.97 | 100 | 631.0 | 451.0 | - | 633.0 | 453.0 |
| 35. | Perfluorooctanesulfonamide (PFOSA) | 10.19 | 50 | 498.0 | 78.0 | 478.0 | - | - |
| 36. | Perfluorotetradecanoic acid (PFTeDA) | 10.21 | 50 | 712.7 | 668.7 | 169.0 | - | - |
| 37. | Perfluorododecanesulfonic acid (PFDoS) | 10.40 | 50 | 698.7 | 80.0 | 99.0 | - | - |
| 38. | N-methyl perfluorooctanesulfonamidoethanol (NMeFOSE) | 11.11 | 500 | 616.0 | 59.0 | - | - | - |
| 39. | N-methyl perfluorooctanesulfonamide (NMeFOSA) | 11.23 | 50 | 511.8 | 219.0 | 169.0 | - | - |
| 40. | N-ethyl perfluorooctanesulfonamidoethanol (NEtFOSE) | 11.41 | 500 | 630.0 | 59.0 | - | - | - |
| 41. | N-ethyl perfluorooctanesulfonamide (NEtFOSA) | 11.52 | 50 | 526.0 | 219.0 | 169.0 | - | - |
| Column | Force C18 (cat.# 9634252) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 1.8 µm | ||||||||||||||||||||||||
| Pore Size: | 100 Å | ||||||||||||||||||||||||
| Guard Column: | Force C18 EXP guard column cartridge 5 mm, 2.1 mm ID, (cat.# 963450252) | ||||||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| Inj. Vol.: | 1 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||||||
| B: | Acetonitrile | ||||||||||||||||||||||||
|
| Detector | Shimadzu 8060 MS/MS |
|---|---|
| Ion Source: | Electrospray |
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | Shimadzu Nexera X2 |
| Sample Preparation | Samples were aliquoted into a 2.0 mL, 9 mm screw-thread polypropylene vials (cat.# 23242) and capped with 9 mm solid-top polyethylene caps (cat.# 23244). |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
In this method, a Force C18 column is implemented using water containing 5 mM ammonium acetate and neat acetonitrile as mobile phases to achieve > 1 minute resolution between PFOS (8.8 min.) and TDCA (5.8 min.) while also resolving TDCA from all other PFAS analytes in a quick, 14-minute cycle time. Even when all three bile acids are analyzed, resolution greater than 1 minute between the bile acids and PFOS is achieved when using ACN but the resolution between TUDCA (RT = 5.5 min.) and ADONA (RT = 5.7 min.) narrows.
But what happens when we switch to methanol as the organic modifier? Now, instead of requiring the monitoring of only one bile acid interference, there are three that need to be resolved from PFOS by at least one minute while also not coeluting with any other PFAS compound per the requirements of EPA 1633. In addition to the EPA 1633 requirements that need to be met, the method needs to be kept as short as possible to not waste valuable instrument time. Let’s look at some method scouting chromatograms using methanol as the organic modifier.
Using a scouting gradient, the 1633 standard and a mixture of bile acids were analyzed and their retention times compared. When using methanol, it is difficult to achieve the one-minute separation that is required for PFOS, TCDCA, and TDCA with only 0.263 minutes between PFOS and the closest eluting bile acid in the provided example. Next, the method was tested again using a longer run time and extending the gradient from 40-75% by two minutes.
These efforts resulted in slightly more resolution between PFOS and the closest eluting bile acid, TCDCA, but at the cost of a longer method cycle time. Several other conditions were tested to meet the requirements of the EPA 1633 document using methanol, but no conditions tested were able to meet all conditions within a reasonable analysis time. Overall, acetonitrile proves to be the superior solvent choice for this analysis of EPA 1633 and is able to achieve all outlined requirements.
