Analysis of Trace Oxygenates in Petroleum-Contaminated Wastewater, Using Purge-and-Trap GC-MS (U.S. EPA Methods 5030B & 8260)
Gasoline and other petroleum-derived fossil fuels consist mainly of compounds that contain only carbon and hydrogen atoms. Oxygenates— ethers and alcohols—contain oxygen atoms in addition to carbon and hydrogen. Oxygenates are added to gasoline to enhance various aspects of its performance as a fuel. Methyl tertiary-butyl ether (MTBE) is the most common fuel oxygenate. MTBE was first introduced into gasoline in 1979, to reduce overall emissions, replace lead, and increase octane rating. In 1992, gasoline with up to 15% MTBE content by volume was used nationally to meet the first federally mandated wintertime reduction of carbon monoxide emission.
With over one million underground fuel tanks in the United States, contamination of ground and surface water with gasoline components, including oxygenates, is a major environmental concern. Storage tanks worldwide potentially will require monitoring and cleanup in the future. The U.S. EPA has not sanctioned any wastewater method specifically for analysis of oxygenates from gasoline. Consequently, environmental laboratories in the U.S. have used a variety of methods to report these analytes, including EPA Methods 8015B, 8021B, and 8260. Regulatory agencies recommend adding tert-butyl alcohol (TBA) to the target list for contaminated sites known to contain MTBE because it is both a breakdown product of MTBE and a gasoline additive in its own right. Identification and quantification of these fuel-derived pollutants in a gasoline-water matrix is a challenging task, because compounds such as MTBE and TBA coelute on many of the GC column stationary phases in capillary gas chromatography columns, and share ions used for identification in gas chromatography-mass spectrometry (GC-MS).
Method 8015B calls for using a flame ionization detector (FID) to match a known chromatographic pattern for gasoline to an unknown sample containing peaks that fall within the gasoline pattern range. This method can be used to identify oxygenates by retention time, but a high probability of misidentifications dictates the need for confirmation on a second column. A second analysis entails additional costs and additional time. Method 8021B was written specifically for analysis of aromatic and halogenated volatiles, and employs a photoionization detector (PID). This is the least reliable of the 3 methods: a PID is very sensitive to double bonds, but is much less sensitive to ether and hydroxy groups. In a test analysis of a gasoline composite standard conducted in our laboratories, use of Method 8021B resulted in false positive identification of a sample component as diisopropyl ether. Confirmation analysis by GC-MS correctly identified the compound as 2-methyl-1-pentene [1].
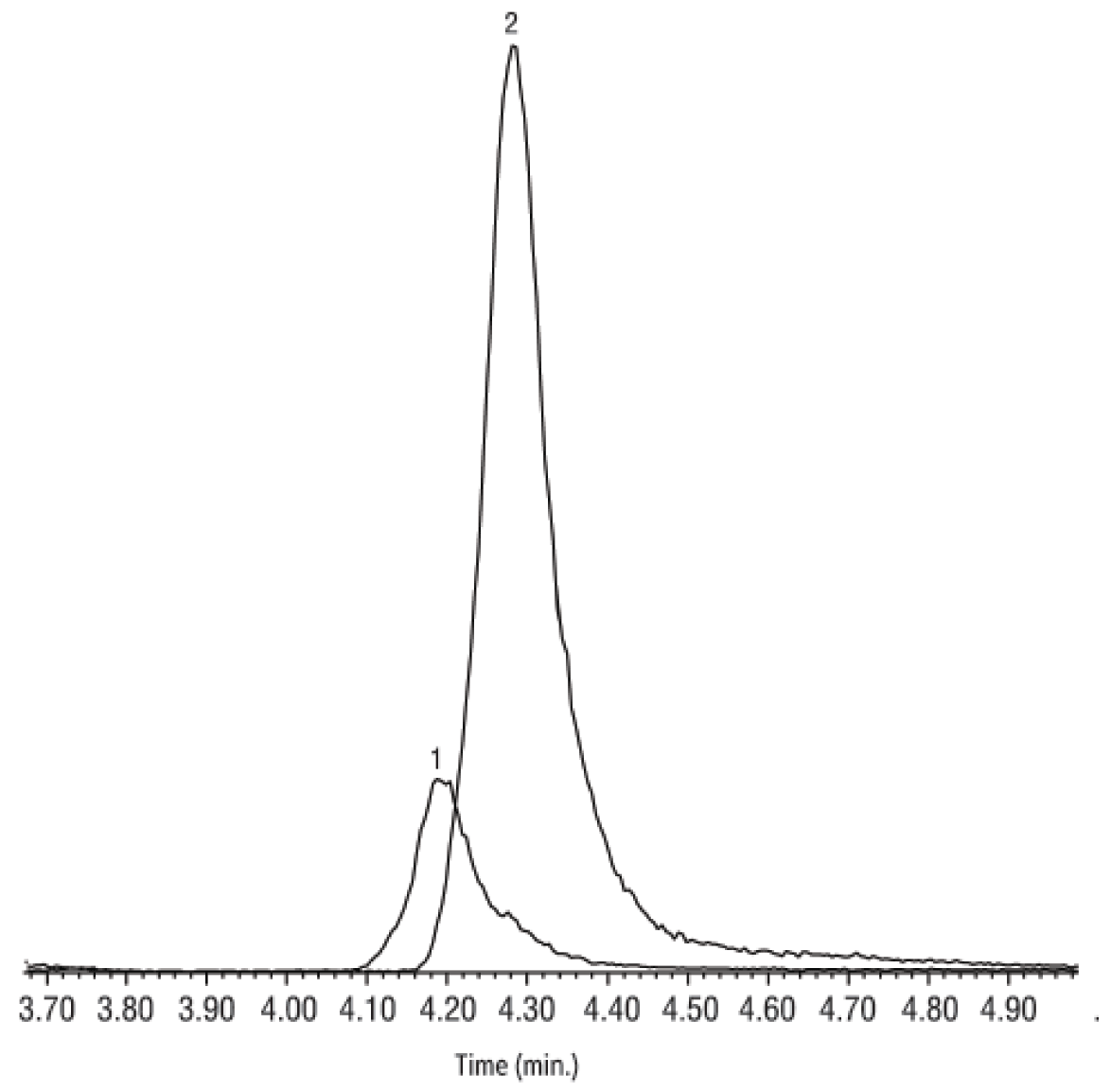
Purge-and-trap GC-MS methods, such as Method 8260, are favored for monitoring oxygenates in gasoline because they ensure a higher level of confidence in the data obtained, compared to GC methods, as illustrated by the 2-methyl-1-pentene example cited above. Under appropriate conditions, GC-MS circumvents the problem of false identification of unresolved constituents and provides positive identifications. Studies conducted by analysts at Lawrence Livermore National Laboratory indicate that GC-MS is the most reliable method of detecting oxygenates in complex gasoline-containing samples, regardless of the concentration of the gasoline (from neat to greatly diluted with groundwater) [2]. Although confidence in the GC-MS approach is well founded, the GC column stationary phase must be carefully chosen and tested to assure that there are no critical coelutions between target oxygenated analytes and other gasoline components, which could result in false positives for the former. Further, careful attention to operating conditions is necessary, to maximize analyte response and optimize resolution between oxygenates and other compounds of interest. TBA and MTBE, for example, must be separated, because they share ions used for identification. Figure 1 is an example of inadequate resolution of these analytes; "624"-type stationary phases are not recommended for oxygenates analysis due to this limitation. Similarly, incomplete resolution of tert-amyl-methyl ether (TAME) and benzene makes an Rtx-Volatiles column inappropriate for this application.
Figure 1: "624"-type stationary phases do not resolve TBA and MTBE adequately for positive identification and reliable quantification.
| Column | Rtx-624, 30 m, 0.25 mm ID, 1.40 µm (cat.# 10968) |
|---|---|
| Standard/Sample | 8260B MegaMix calibration mix kit (cat.# 30475) |
| California oxygenates mix (cat.# 30465) | |
| VOA calibration mix #1 (ketones) (cat.# 30006) | |
| 8260A Surrogate mix (cat.# 30240) | |
| 8260 Internal standard mix (cat.# 30074) | |
| Injection | purge and trap split (split ratio 25:1) |
| Inj. Temp.: | 250 °C |
| Purge and Trap | |
| Instrument: | O.I. Analytical 4560 with 4551A Autosampler |
| Trap Type: | #10 (Tenax/silica gel/carbon molecular sieve) |
| Purge: | 11 min @ 20 °C, flow 38 mL/min |
| Desorb Preheat Temp.: | 150 °C |
| Desorb: | 1.0 min @ 190 °C, flow 32 mL/min |
| Bake: | 10 min @ 210 °C |
| Transfer Line Temp.: | 110 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 7 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.3 mL/min |
| Dead Time: | 1.47 min @ 35 °C |
| Detector | Agilent 5971A GC/MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Tune Type: | PFTBA/BFB |
| Scan Range: | 35-260 amu |
| Notes | Sample size: 10 mL Sample temp: 40 °C Water Management: 110 °C purge, 0 °C desorb, 240 °C bake 6-Port Valve: 110 °C Sparge Mount: 45 °C Valve Manifold: 50 °C Other Conditions: prepurge, preheat, dry purge OFF |
| Acknowledgement | Purge & trap courtesy of O.I. Analytical |
We evaluated the Rtx-VMS stationary phase for analysis of oxygenates, verifying passing criteria by following EPA Method 5030B (with modification) and Method 8260. Samples of non-oxygenated gasoline, spiked with low levels of oxygenates, enabled us to determine if operating conditions were appropriate for separating and detecting these target compounds in the presence of high concentrations of other gasoline components. Purge-and-trap conditions in Method 5030B were modified to address the oxygenates in the samples: to increase responses for the oxygenates, we used a 40 °C purge temperature, rather than the ambient temperature suggested. GC oven conditions were optimized to eliminate coelutions of ion-sharing analytes.
The GC-MS system was tuned using a 25 ng standard of 4-bromofluorobenzene (BFB). Commonly analyzed compounds on the Method 8260 target list were added to the calibration mix, along with internal standards, ethers, and TBA. Methanol and ethanol were not added to these samples; these low molecular weight compounds require higher purge temperatures or are performed using static headspace techniques.
Method 8260B does not specifically assign internal standards to target compounds. Therefore, we used the internal standards and corresponding analytes listed in Table 6 of Method 8260 [3]. This allowed us to measure response factors (RF) against established internal standards. We also incorporated the Method 8260 surrogates, and the characteristic primary ions for quantification, into our experimental design. The curve was calibrated using mean response factors, as outlined in Method 8260, section 7.6.2.1. Minimum mean response factors for the volatile system performance check compounds (SPCC), as described in the method, are listed in Table I. These values confirm acceptable performance by the purge-and-trap concentrator. Note that the 40 °C purge temperature used to increase responses for the oxygenates also will increase responses for the SPCC compounds.
Table I: System performance check compound (SPCC) response factor criteria for all columns under specific conditions.
| EPA Criterion (Minimum Mean RF) |
Rtx-VMS Column (RF) | |
|---|---|---|
| Pentafluorobenzene (IS) | ||
| Chloromethane (SPCC) | >0.10 | 0.59 |
| 1,1-Dichloroethane (SPCC) | >0.10 | 0.64 |
| Chlorobenzene-d5 (IS) | ||
| Chlorobenzene (SPCC) | >0.30 | 1.06 |
| Bromoform (SPCC) | >0.10 | 0.32 |
| 1,4-Dichlorobenzene-d4 (IS) | ||
| 1,1,2,2-Tetrachloroethane (SPCC) |
>0.30 | 0.71 |
After the system passed the SPCC evaluation, the calibration check compounds (CCC) were used to verify the validity of the calibration. Relative standard deviations (%RSD) for these compounds must be less than 15% (Table II). The internal standards in Table III were used for calculating RFs, %RSDs, and % recoveries (%Recov.) of target analytes, as shown. Oxygenates were calculated relative to internal standard methyl-d3-tert-butyl ether, to account for differences in purging efficiency specific to the ethers.
Table II: Calibration check compound (CCC) relative standard deviation criteria for all columns under specific conditions.
| EPA Criterion (%RSD) | Rtx-VMS Column (%RSD) | |
|---|---|---|
| Pentafluorobenzene (IS) | ||
| 1,1-Dichlorothene (CCC) | <15 | 11.4 |
| Chloroform (CCC) | <15 | 5.5 |
| 1,4-Difluorobenzene (IS) | ||
| 1,2-Dichloropropane (CCC) | <15 | 4.9 |
| Toluene (CCC) | <15 | 3.0 |
| Chlorobenzene-d5 (IS) | ||
| Ethylbenzene (CCC) | <15 | 5.5 |
| 1,4-Dichlorobenzene-d4 (IS) | ||
| 1,1,2,2-Tetrachloroethane (SPCC) | <15 | 8.6 |
Table III: Internal standards with corresponding analytes assigned for quantification.
| Methyl-d3-tert-butyl ether (IS) | Pentafluorobenzene (IS) | 1,4-Difluorobenzene (IS) | Chlorobenzene-d5 (IS) | 1,4-Dichlorobenzene-d4 (IS) |
|---|---|---|---|---|
| methyl-tert-butyl ether | chloromethane (SPCC) | 1,2-dichloroethane-d4 (SS) | chlorobenzene (SPCC) | 1,1,2,2-tetrachloroethane (SPCC) |
| tert-butyl alcohol (x5)* | 1,1-dichlorothene (CCC) | 1,2-dichloropropane (CCC) | ethylbenzene (CCC) | naphthalene |
| diisopropyl ether | acetone (x2.5)* | toluene-d8 (SS) | bromoform (SPCC) | |
| ethyl-tert-butyl ether | 1,1-dichloroethane (SPCC) | toluene (CCC) | ||
| tert-amyl-methyl ether | chloroform (CCC) | bromofluorobenzene (SS) | ||
| dibromofluoro-methane (SS) |
*Compound added at 5 times or 2.5 times the concentration of other target analytes.
Purge-and-Trap Procedures
Table IV summarizes the purge-and-trap conditions used for recovering oxygenates from gasoline. Samples were heated using the Infra-Sparge sample heater on an O.I. 4560 concentrator. The minimum purge temperature effective for detecting TBA at a concentration of 25 ppb in 10 mL of water was 40 °C. Purge flow rate was carefully adjusted to 38 mL/min; lower flows dramatically affect recovery of the brominated compounds, higher flows contribute to analyte breakthrough and excessive water retention on the trap.
Table IV: Purge-and-trap conditions for recovering oxygenates from gasoline (O.I. 4560 concentrator).
| Trap: | #10 (Tenax/silica gel/carbon molecular sieve) |
| Purge time: | 11 min |
| Purge flow rate: | 38 mL/min |
| Desorb flow rate: | 32 mL/min |
| Desorb time: | 1.0 min |
| Bake time: | 10 min |
| Sample size: | 10 mL |
| Water management: | 110 °C purge, 0 °C desorb, 240 °C bake |
| Split ratio: | 1:25 |
| Temperatures: | |
| Sample: | 40 °C |
| Trap: | 20 °C purge, 190 °C desorb, 210 °C bake |
| 6-Port valve: | 110 °C |
| Transfer line: | 110 °C |
| Sparge mount: | 45 °C |
| Desorb preheat: | 150 °C |
| Valve manifold: | 50 °C |
| Other conditions: | Pre-purge, pre-heat, dry purge OFF |
Conditions suggested by O.I. Analytical.
Analytical Procedures
An Agilent 5890 Series II GC coupled with an Agilent 5971A GC-MS detector fitted with a K&M electron multiplier was used for the analysis. Helium carrier gas was adjusted to 1.3 mL/min constant flow. The oven temperature program was optimized as follows: 35 °C (hold 7 min) to 90 °C @ 4 °C/min (hold 0 min), to 220 °C @ 45 °C/min (hold 1 min). Analysis time was 25 minutes; cycle time was 30 minutes. The MS was set for full scan from 35 amu to 260 amu and was initially tuned with or PFTBA calibration gas, followed by a BFB tune check. A 30 m x 0.25 mm x 1.4 μm Rtx-VMS column (cat.# 19915) was used for the separations.
Standards
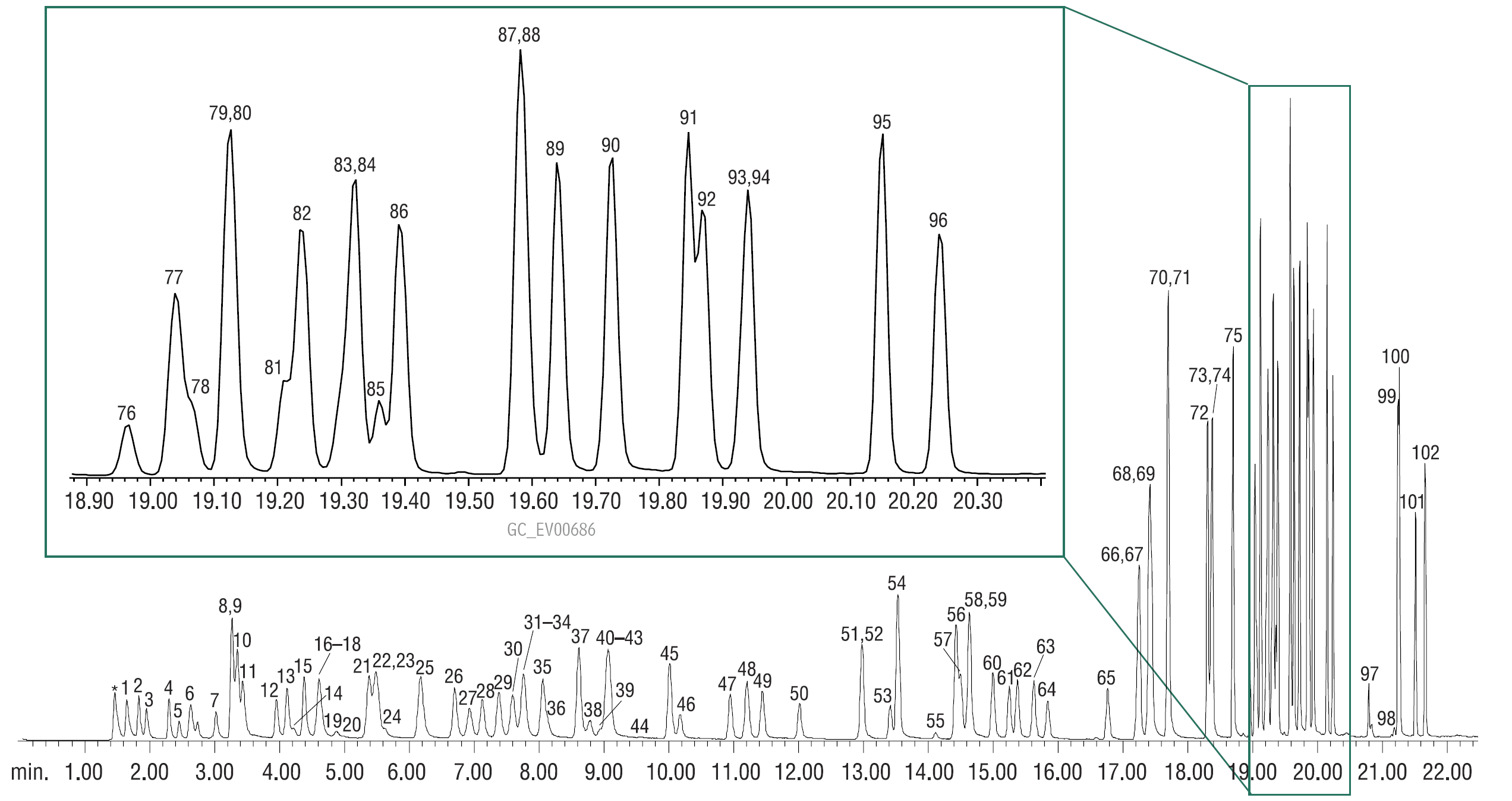
The five points of the calibration were 5, 10, 20, 40, and 80 ppb, with internal standards (IS) and surrogate standards (SS) added to each calibration standard at 20 ppb. Intermediate standards were made separately for each calibration point, to maintain equal amounts of methanol added to the 10 mL volume of water. Samples were spiked and were transferred to the concentrator by hand. Calibration was performed using the analyte list in Table III, but all compounds shown in Figure 2 were added in order to check for critical coelutions between oxygenates and Method 8260B target compounds. Calibration verification standards (CVS) were added at 10 ppb; recoveries were within 20% of expected values. Two blanks were analyzed, followed by a 5 ppb QC standard to verify recoveries at the low point of the curve [4]. Reference materials used are listed in Table V.
Figure 2: Detecting oxygenates in gasoline-water samples: 80 ppb each analyte.
| Column | Rtx-VMS, 30 m, 0.25 mm ID, 1.4 µm (cat.# 19915) |
|---|---|
| Standard/Sample | 8260B MegaMix calibration mix kit (cat.# 30475) |
| California oxygenates mix (cat.# 30465) | |
| VOA calibration mix #1 (ketones) (cat.# 30006) | |
| 8260A surrogate mix (cat.# 30240) | |
| 8260 internal standard mix (cat.# 30074) | |
| Injection | purge and trap split (split ratio 25:1) |
| Inj. Temp.: | 250 °C |
| Purge and Trap | |
| Instrument: | O.I. Analytical 4560 with 4551A Autosampler |
| Trap Type: | #10 (Tenax/silica gel/carbon molecular sieve) |
| Purge: | 11 min @ 20 °C, flow 38 mL/min |
| Desorb Preheat Temp.: | 150 °C |
| Desorb: | 1.0 min @ 190 °C, flow 32 mL/min |
| Bake: | 10 min @ 210 °C |
| Transfer Line Temp.: | 110 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 7 min) to 90 °C at 4 °C/min to 220 °C at 45 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.3 mL/min |
| Dead Time: | 1.47 min @ 35 °C |
| Detector | Agilent 5971A GC-MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Tune Type: | PFTBA/BFB |
| Scan Range: | 35-260 amu |
| Notes | Sample Size: 10 mL Sample Temp: 40 °C Water Management: 110 °C purge, 0 °C desorb, 240 °C bake 6-Port Valve: 110 °C Sparge Mount: 45 °C Valve Manifold: 50 °C Other Conditions: prepurge, preheat, dry purge OFF |
| Acknowledgement | Purge & trap courtesy of O.I. Analytical |
Table V: Calibration mixes and other reference materials for oxygenates analysis.
| Reference Material | Restek cat.# | Lot# |
|---|---|---|
| Internal standard and surrogate standard mix |
cat.# 30240 | lot# A025538 |
| custom | lot# 03010401s | |
| cat.# 30074 | lot# A022472 | |
| Calibration mix | cat.# 30475 | lot# A020908 |
| cat.# 30465 | lot# A024826 | |
| cat.# 30006 | lot# A024175 | |
| cat.# 30042 | lot# A024616 | |
| QC second source calibration mix | custom | lot# A025888 |
| Unleaded gasoline (unweathered) |
cat.# 30096 | lot# A022384 |
| Non-oxygenated unleaded gasoline (unweathered) |
custom | lot# OFR-TK253 |
Gasoline-Spiked Samples
Analysis of the 5 ppb QC standard was followed by analysis of a 1 ppm non-oxygenated gasoline standard, then by analysis of the non-oxygenated standard with 5 ppb of each target compound added. Recoveries for the 5 ppb oxygenate standards were calculated from this high concentration gasoline matrix. The final standard analyzed was 1 ppm (unweathered) gasoline. All samples were spiked with the appropriate IS and SS. The calibration curve passed EPA 8260 criteria for response factors and relative standard deviations. Only two compounds in the test set showed poor response: acetone and TBA.
Results
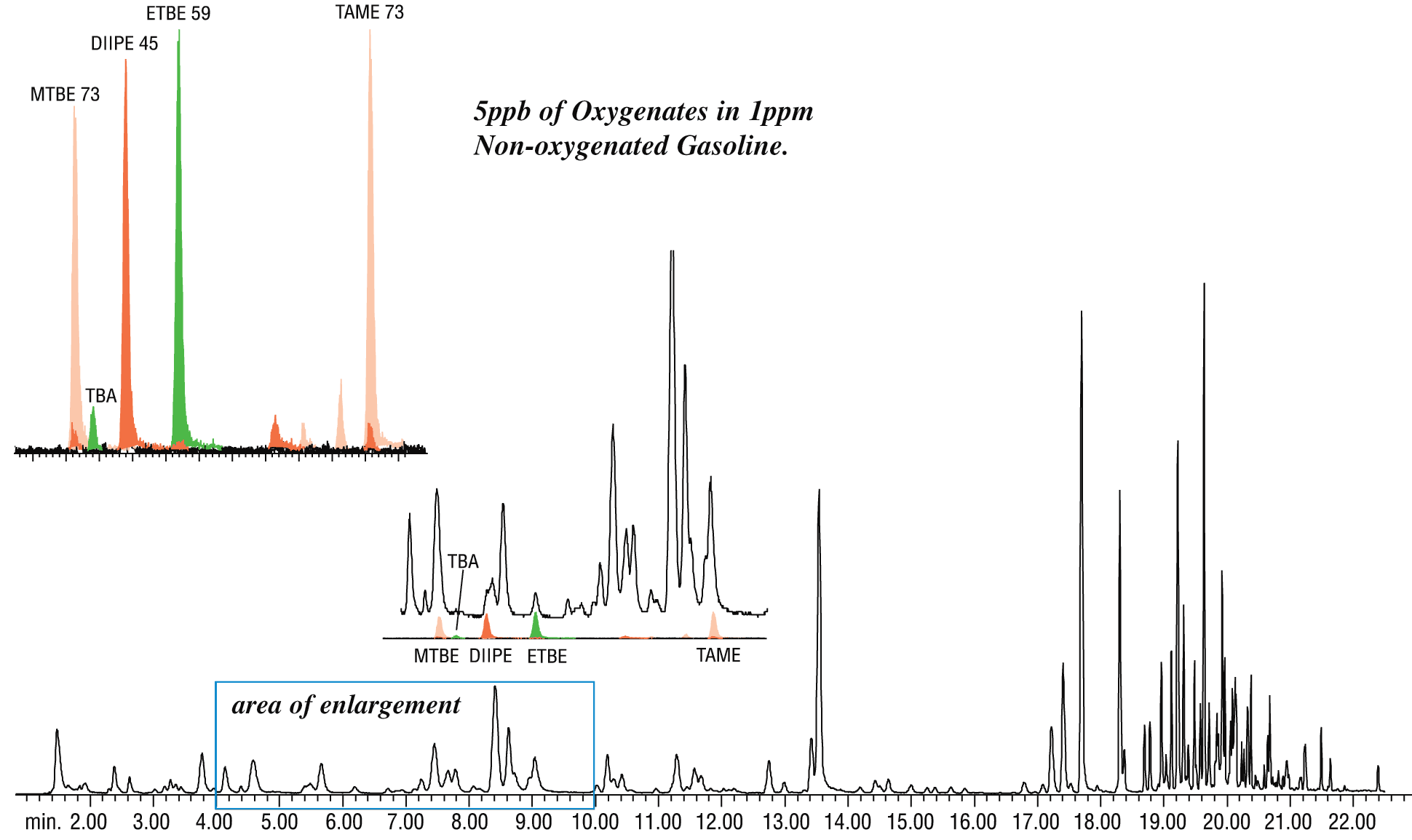
Table VI summarizes results. All oxygenates were resolved from other target compounds and potentially interfering gasoline components. TBA and MTBE were well resolved using the 35 °C GC oven starting temperature. Figure 3 represents a TIC of 1 ppm non-oxygenated unleaded gasoline spiked with 5 ppb of oxygenates. The inset is an extracted ion chromatogram showing oxygenates are recovered without interference from the gasoline matrix.
Table VI: Efficient recovery of ppb oxygenates, using an Rtx-VMS column.
| Reference Material | Restek cat.# | Lot# |
|---|---|---|
| Internal standard and surrogate standard mix |
cat.# 30240 | lot# A025538 |
| custom | lot# 03010401s | |
| cat.# 30074 | lot# A022472 | |
| Calibration mix | cat.# 30475 | lot# A020908 |
| cat.# 30465 | lot# A024826 | |
| cat.# 30006 | lot# A024175 | |
| cat.# 30042 | lot# A024616 | |
| QC second source calibration mix | custom | lot# A025888 |
| Unleaded gasoline (unweathered) |
cat.# 30096 | lot# A022384 |
| Non-oxygenated unleaded gasoline (unweathered) |
custom | lot# OFR-TK253 |
*Compound added at 5 times or 2.5 times the concentration of other target analytes.
< LOD = below limit of detection limit; ND = not detected.
Column: Rtx-VMS, 30 m x 0.25 mm x 1.4 μm (cat.# 19915)
Conditions: given in text
Figure 3: GC/MS with an Rtx-VMS column allows reliable identification of 5 ppb oxygenates added to 1 ppm gasoline.
| Column | Rtx-VMS, 30 m, 0.25 mm ID, 1.40 µm (cat.# 19915) |
|---|---|
| Standard/Sample | 8260B MegaMix calibration mix kit (cat.# 30475) |
| California oxygenates mix (cat.# 30465) | |
| VOA calibration mix #1 (ketones) (cat.# 30006) | |
| 502.2 Calibration mix #1 (gases) (cat.# 30042) | |
| Unleaded gasoline: unweathered (cat.# 30096) | |
| 8260A Surrogate mix (cat.# 30240) | |
| 8260 Internal standard mix (cat.# 30074) | |
| Non-oxygenated unleaded gasoline (custom) | |
| Injection | purge and trap split (split ratio 25:1) |
| Inj. Temp.: | 250 °C |
| Purge and Trap | |
| Instrument: | O.I. 4560 Purge and Trap Concentrator |
| Trap Type: | #10 Tenax/silica gel/carbon molecular sieve |
| Purge: | 11 min @ 20 °C, flow 38 mL/min |
| Desorb: | 1.0 min @ 190 °C, flow 32 mL/min |
| Bake: | 10 min @ 210 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 7 min) to 90 °C at 4 °C/min to 220 °C at 45 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.3 mL/min |
| Dead Time: | 1.47 min @ 35 °C |
| Detector | MS |
|---|---|
| Mode: | Scan |
| Tune Type: | PFTBA/BFB |
| Scan Range: | 35-260 amu |
| Instrument | Aglient 5971A GC/MS |
| Notes | 10 mL sample @ 40°C |
| Acknowledgement | Purge and Trap courtesy of O.I. Analytical. |
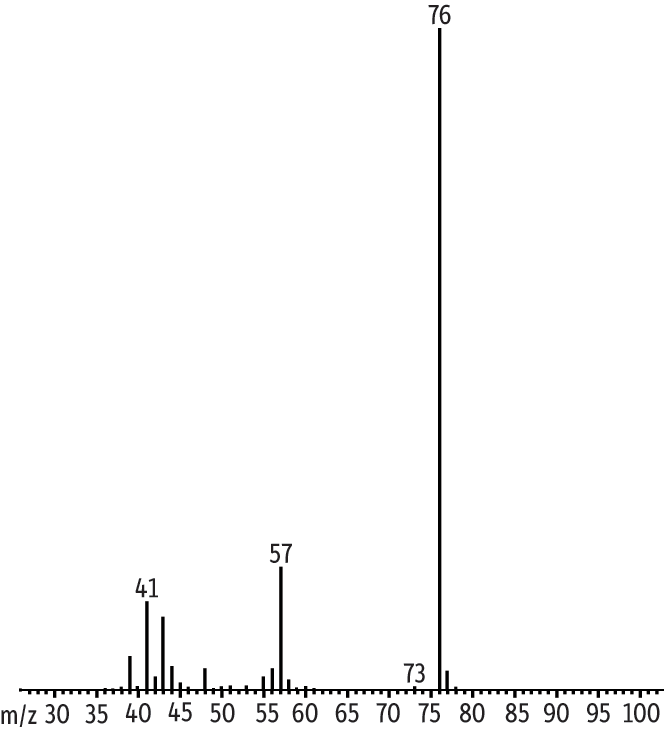
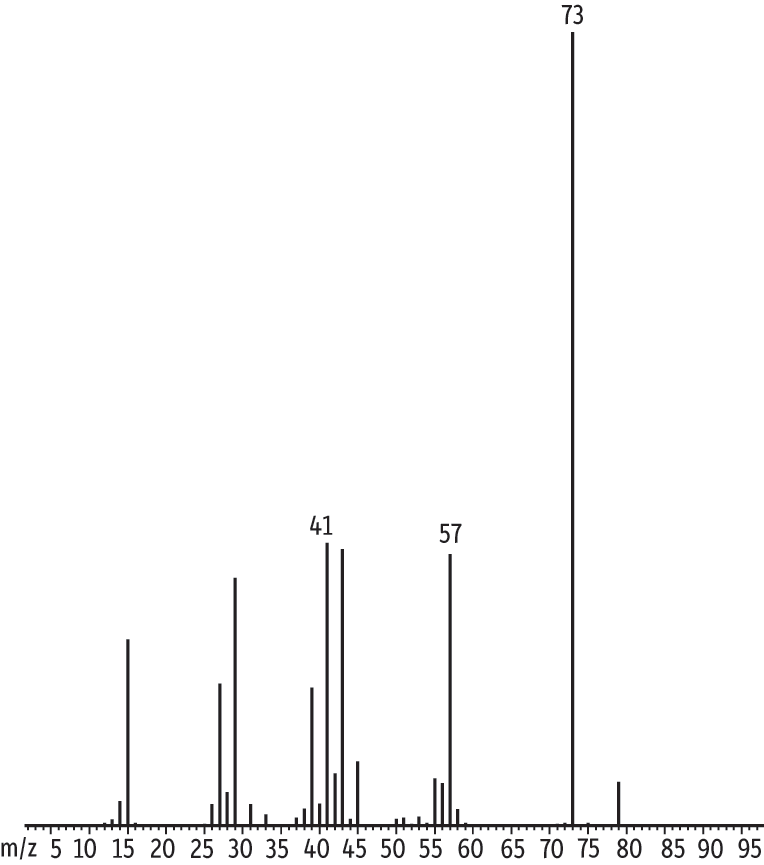
The internal standard methyl-d3 tert-butyl ether was added to compensate for variations in purging efficiency specific to the ethers. After the data were acquired we observed a small ion 73 component as part of the IS mass spectrum (Figure 4). MTBE and the IS share retention time and MTBE produces ion 73. Our initial thought was to discard the data, but we determined the distribution of the relative abundance of ion 73 to ion 76 for the IS was between 0.3% and 0.5%. This would affect calculated concentrations of MTBE by no more than 2%. Analysts in environmental laboratories can decide if this is an acceptable degree of error. Some laboratories use TBA-d9 instead, which accounts for variations common to alcohols, but not to ethers.
Figure 4: MTBE and internal standard methyl-d3-tert-butyl ether share identification ion 73.
MTBE-d3
MTBE
| Column | Rtx-VMS, 30 m, 0.25 mm ID, 1.4 µm (cat.# 19915) |
|---|---|
| Standard/Sample | Custom |
| Injection | purge and trap split (split ratio 25:1) |
| Inj. Temp.: | 250 °C |
| Purge and Trap | |
| Instrument: | O.I. Analytical 4560 with 4551A Autosampler |
| Trap Type: | #10 (Tenax/silica gel/carbon molecular sieve) |
| Purge: | 11 min @ 20 °C, flow 38 mL/min |
| Desorb Preheat Temp.: | 150 °C |
| Desorb: | 1.0 min @ 190 °C, flow 32 mL/min |
| Bake: | 10 min @ 210 °C |
| Transfer Line Temp.: | 110 °C |
| Oven | |
| Oven Temp.: | 35 °C (hold 7 min) to 90 °C at 4 °C/min to 220 °C at 45 °C/min (hold 1 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.3 mL/min |
| Dead Time: | 1.47 min @ 35 °C |
| Detector | Agilent 5971A GC/MS |
|---|---|
| Transfer Line Temp.: | 280 °C |
| Tune Type: | PFTBA/BFB |
| Scan Range: | 35-260 amu |
| Notes | Sample size: 10 mL Sample temp: 40 °C Water management: 110 °C purge, 0 °C desorb, 240 °C bake 6-Port valve: 110 °C Sparge mount: 45 °C Valve manifold: 50 °C Other Conditions: prepurge, preheat, dry purge OFF |
| Acknowledgement | Purge & trap courtesy of O.I. Analytical |
Conclusion
With an expanding target list and difficult sample matrixes, such as petroleum distillates, extreme care must be taken, even with GC-MS, to assure correct identification of oxygenates in the presence of interfering analytes. Under the conditions used here, an Rtx-VMS column is suitable for analyzing low levels of oxygenates in the presence of other gasoline components.