Excellent Resolution of EU-Regulated Azo Dye Aryl Amines by GC-MS on the Rxi-35Sil MS GC Column
Abstract
A high-speed GC-MS method was developed for the analysis of carcinogenic aryl amines derived from reductive cleavage of certain azo dyes using the Rxi-35Sil MS column. The use of azo dyes that break down to form these amines in clothing is a potential exposure risk and is regulated in the European Union (EU) under the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulation instated by (EC) 1907/2006. Additionally, certification under OEKO-TEX Standard 100 requires textiles to comply with (EC) 1907/2006. The optimal selectivity, high efficiency, and inertness of the Rxi-35Sil MS column make it an ideal choice for high-throughput GC-MS analysis of trace-level aryl amines.
Azo Dyes and Products of Reductive Cleavage
Azo dyes were among the first chemical dyes characterized and synthesized on a large scale. Today, they are widely used in food products, cosmetics, artist paints, and textiles. The name of this class of dyes is derived from the common presence of the azo functional group linking two aromatic moieties. Azo dyes are readily prepared by azo coupling of aryl primary amines with activated aromatic substrates like anilines and phenols. The azo linkage creates a fully conjugated system and its absorbance in the visible spectrum gives it intense color. The color of the final azo dye product is controlled by the extent of conjugation and chemical substitution of the coupling partners. Figure 1 shows the structural elements of some typical azo dyes, note the common R1-N=N-R2 azo group.
Figure 1: Structures of some typical azo dyes, note the common azo group linkage of the aryl units.
The azo functional group is prone to chemical reduction, which results in cleavage of the N=N bond and production of the corresponding amines. This reductive cleavage process can occur in textiles colored with azo dyes when the article is exposed to human sweat, saliva, or symbiotic bacteria that inhabit human skin [1,2]. In the case of some specific azo dyes, the aryl amines produced by reductive cleavage are carcinogenic and have also caused contact dermatitis in sensitive individuals [3,4]. Figure 2 shows reductive cleavage of Direct Blue 25, an azo dye that produces the listed aryl amine 3,3’-dimethylbenzidine.
Figure 2: Reductive cleavage of Direct Blue 25, an azo dye that produces the listed 3,3’-dimethylbenzidine upon reductive cleavage.
Due to their human exposure risk, the EU has regulated azo dyes producing certain carcinogenic aryl amines under the REACH system instated by (EC) 1907/2006 [5]. To test for the presence of regulated azo dyes, textile items are extracted with refluxing chlorobenzene and the extract is subjected to reduction with sodium dithionite at pH 6 according to standard method EN 14362-1:2012 [6]. The reduced extract is analyzed by GC-MS and textiles producing more than 30 mg/kg of any single listed aryl amine are considered to be in violation. In order for a textile item to be certified free of harmful substances under OEKO-TEX Standard 100, an important, comprehensive, and internationally recognized standard, it must comply with (EC) 1907/2006 [7].
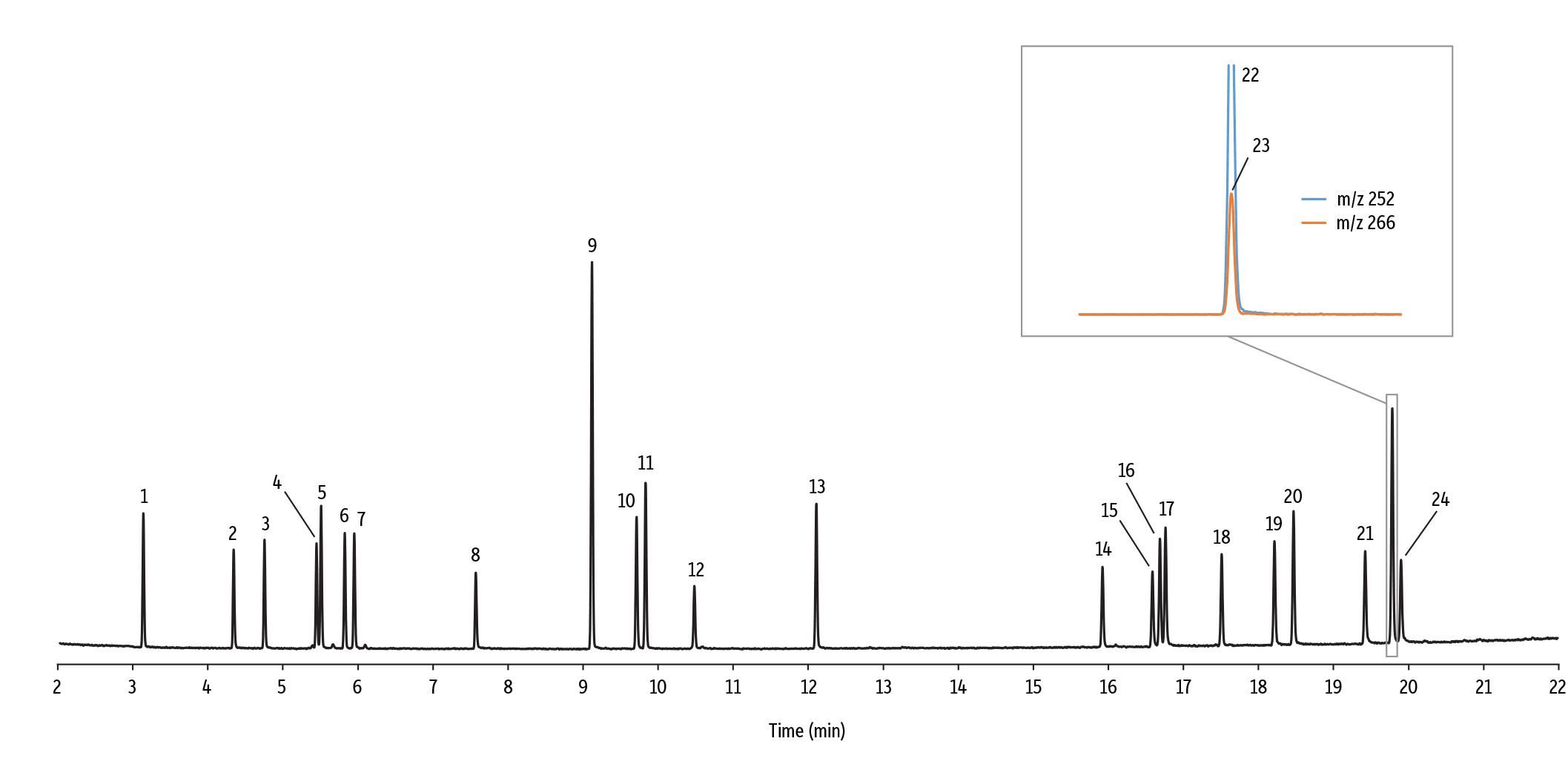
EU-Listed Azo Dye Aryl Amines Are Separated with High Chromatographic Resolution
It is well established that MS is a powerful tool for the deconvolution of coeluting chromatographic peaks, but there are certain pairs of analytes that can only be independently quantitated when they are well resolved from each other, for example, positional isomers of the same compound. Positional isomers of aryl amines differ only in the relative positions of substituents around the benzene ring. As a consequence, their electron ionization (EI) mass spectra contain ions of the same m/z and are virtually impossible to distinguish from each other. If two positional isomers coelute they cannot be deconvoluted because there are no fragment ions unique to one isomer or the other. Chromatographic separation of positional isomers requires a GC column with the proper selectivity and high efficiency.
Figure 3 demonstrates that even under very general GC conditions, such as those recommended by EN 14362-1:2012, the Rxi-35Sil MS column separates not only 20 of the 22 EU-listed aryl amines but also positional isomers of 4-chloro-o-toluidine and 4-aminobiphenyl. While only the 4-substituted compounds are listed, detection of the other positional isomers can provide structural information about the offending azo dye that produced them.
Figure 3: The Rxi-35Sil MS column chromatographically separates 20 of the 22 EU-listed azo dye aryl amines as well as positional isomers under the generic standard method conditions in EN 14362-1:2012.
| Peaks | tR (min) | Conc. (mg/mL) | Molecular Ion (m/z) | |
|---|---|---|---|---|
| 1. | o-Toluidine | 3.15 | 10 | 107 |
| 2. | o-Anisidine | 4.36 | 10 | 123 |
| 3. | 4-Chloroaniline | 4.77 | 10 | 127 |
| 4. | p-Cresidine | 5.46 | 10 | 137 |
| 5. | 2,4,5-Trimethylaniline | 5.52 | 10 | 135 |
| 6. | 3-Chloro-o-toluidine | 5.84 | 10 | 141 |
| 7. | 4-Chloro-o-toluidine | 5.96 | 10 | 141 |
| 8. | 2,4-Diaminotoluene | 7.58 | 10 | 122 |
| 9. | 2,4,5-Trichloroaniline (IS) | 9.13 | 30 | 196 |
| 10. | 2-Naphthylamine | 9.72 | 10 | 143 |
| 11. | 2-Aminobiphenyl | 9.84 | 10 | 169 |
| 12. | 2-Amino-4-nitrotoluene | 10.49 | 10 | 152 |
| Peaks | tR (min) | Conc. (mg/mL) | Molecular Ion (m/z) | |
|---|---|---|---|---|
| 13. | 4-Aminobiphenyl | 12.12 | 10 | 169 |
| 14. | p-Aminoazobenzene | 15.93 | 10 | 197 |
| 15. | 4,4'-Oxydianiline | 16.60 | 10 | 200 |
| 16. | 4,4'-Diaminodiphenylmethane | 16.70 | 10 | 198 |
| 17. | Benzidine | 16.77 | 10 | 184 |
| 18. | o-Aminoazotoluene | 17.52 | 10 | 225 |
| 19. | 3,3'-Dimethyl-4,4'-diaminodiphenylmethane | 18.23 | 10 | 226 |
| 20. | 3,3'-Dimethylbenzidine | 18.48 | 10 | 212 |
| 21. | 4,4’-Thiodianiline | 19.43 | 10 | 216 |
| 22. | 3,3'-Dichlorobenzidine | 19.79 | 10 | 252 |
| 23. | 4,4'-Methylenebis(2-chloroaniline) | 19.79 | 10 | 266 |
| 24. | 3,3'-Dimethoxybenzidine | 19.91 | 10 | 244 |
| Column | Rxi-35Sil MS, 30 m, 0.25 mm ID, 0.25 µm (cat.# 13823) |
|---|---|
| Standard/Sample | AccuStandard carcinogenic aryl amine mix (AE-000-49-R1) |
| Diluent: | Ethyl acetate |
| Conc.: | 10 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Premium 4 mm Precision liner w/wool (cat.# 23305) |
| Inj. Temp.: | 275 °C |
| Oven | |
| Oven Temp.: | 100 °C to 320 °C at 9.5 °C/min (hold 5 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 330 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | Inert | ||||||||
| Drawout Plate: | 3 mm ID | ||||||||
| Source Temp.: | 250 °C | ||||||||
| Quad Temp.: | 180 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Solvent Delay Time: | 2.0 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||
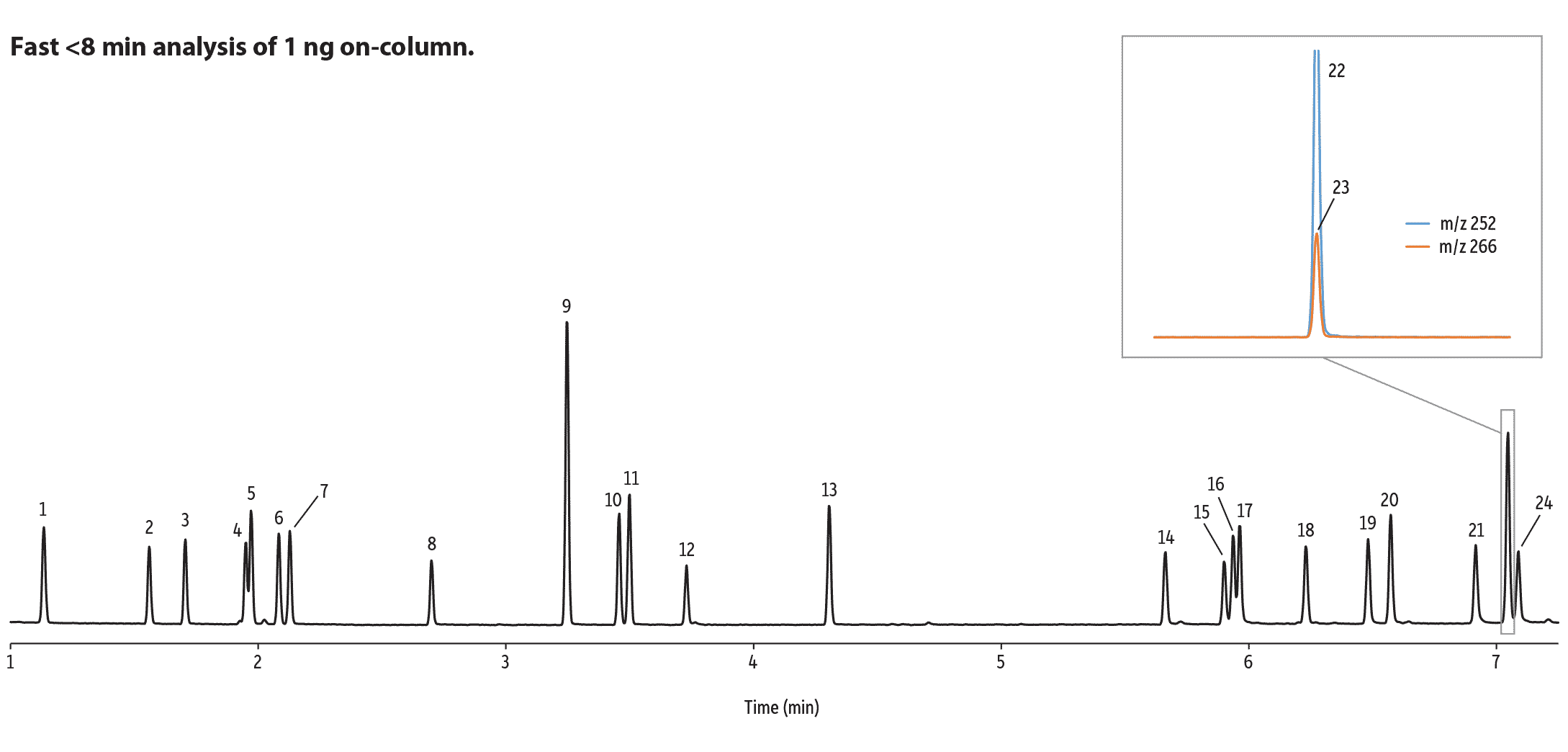
A Short Rxi-35Sil MS Column Offers a Dramatic Reduction in Analysis Time
Because the Rxi-35Sil MS column offers very high chromatographic efficiency and favorable selectivity towards aryl amines, a 15 m column can be used instead of a 30 m column—a substitution that gives a two-fold increase in speed. Using a 15 m x 0.25 mm x 0.25 µm Rxi-35Sil MS column and optimized GC conditions, the high-speed GC-MS analysis of EU-listed azo dye aryl amines sacrifices very little resolution, while maintaining the ruggedness of a 0.25 mm ID column using helium carrier gas. Excellent column inertness results in good peak shape and response for the azo dye aryl amines, even at 1 ng or less on column. Figure 4 shows how a rapid GC-MS analysis can be employed while maintaining a high level of separation performance. High-speed analysis conditions, along with a 15 m Rxi-35Sil MS column, increase analytical throughput and overall laboratory productivity.
Figure 4: The efficiency, selectivity, and inertness of the Rxi-35Sil MS column allow for the analysis of 21 EU-listed azo dye aryl amines in under 8 minutes with minimal sacrifice of separation performance.
| Peaks | tR (min) | Conc. (mg/mL) | Molecular Ion (m/z) | |
|---|---|---|---|---|
| 1. | o-Toluidine | 1.14 | 10 | 107 |
| 2. | o-Anisidine | 1.56 | 10 | 123 |
| 3. | 4-Chloroaniline | 1.71 | 10 | 127 |
| 4. | p-Cresidine | 1.95 | 10 | 137 |
| 5. | 2,4,5-Trimethylaniline | 1.97 | 10 | 135 |
| 6. | 3-Chloro-o-toluidine | 2.08 | 10 | 141 |
| 7. | 4-Chloro-o-toluidine | 2.13 | 10 | 141 |
| 8. | 2,4-Diaminotoluene | 2.70 | 10 | 122 |
| 9. | 2,4,5-Trichloroaniline (IS) | 3.25 | 30 | 197 |
| 10. | 2-Naphthylamine | 3.46 | 10 | 143 |
| 11. | 2-Aminobiphenyl | 3.50 | 10 | 169 |
| 12. | 2-Amino-4-nitrotoluene | 3.73 | 10 | 152 |
| Peaks | tR (min) | Conc. (mg/mL) | Molecular Ion (m/z) | |
|---|---|---|---|---|
| 13. | 4-Aminobiphenyl | 4.31 | 10 | 169 |
| 14. | p-Aminoazobenzene | 5.66 | 10 | 197 |
| 15. | 4,4'-Oxydianiline | 5.90 | 10 | 200 |
| 16. | 4,4'-Diaminodiphenylmethane | 5.94 | 10 | 198 |
| 17. | Benzidine | 5.96 | 10 | 184 |
| 18. | o-Aminoazotoluene | 6.23 | 10 | 225 |
| 19. | 3,3'-Dimethyl-4,4'-diaminodiphenylmethane | 6.48 | 10 | 226 |
| 20. | 3,3'-Dimethylbenzidine | 6.57 | 10 | 212 |
| 21. | 4,4’-Thiodianiline | 6.92 | 10 | 216 |
| 22. | 3,3'-Dichlorobenzidine | 7.05 | 10 | 252 |
| 23. | 4,4'-Methylenebis(2-chloroaniline) | 7.05 | 10 | 266 |
| 24. | 3,3'-Dimethoxybenzidine | 7.09 | 10 | 244 |
| Column | Rxi-35Sil MS, 15 m, 0.25 mm ID, 0.25 µm (cat.# 13820) |
|---|---|
| Standard/Sample | AccuStandard carcinogenic aryl amine mix (AE-000-49-R1) |
| Diluent: | Ethyl acetate |
| Conc.: | 10 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Premium 4 mm Precision liner w/wool (cat.# 23305) |
| Inj. Temp.: | 275 °C |
| Oven | |
| Oven Temp.: | 100 °C to 320 °C at 27 °C/min (hold 1.75 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 330 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | Inert | ||||||||
| Drawout Plate: | 3 mm ID | ||||||||
| Source Temp.: | 250 °C | ||||||||
| Quad Temp.: | 180 °C | ||||||||
| Electron Energy: | 70 eV | ||||||||
| Solvent Delay Time: | 2.0 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||
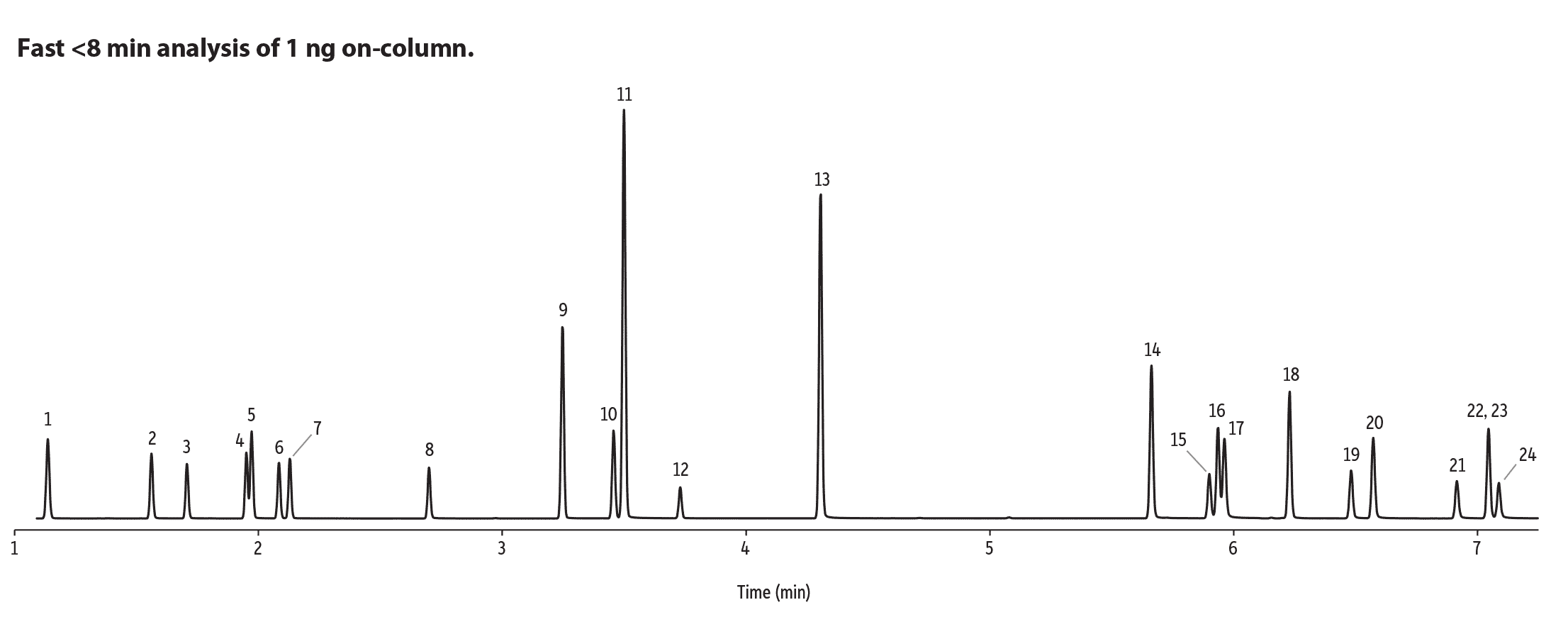
GC-MS-SIM on an Rxi-35Sil MS Column Is Ideal for Routine Analysis of EU Listed Azo Dye Aryl Amines
A GC-MS method employing selected ion monitoring (SIM) mode has the benefit of increasing detector sensitivity and, in turn, decreasing the lower limits of quantitation and detection. Dedicated ion monitoring also helps define narrow chromatographic peaks when using high-speed GC analysis. The use of SIM mode requires that analytes produce unique high m/z ions that ideally are high in abundance. The azo dye aryl amines are good candidates for GC-MS-SIM because they produce high-intensity molecular ions under electron ionization. The molecular ions are highly unique to their corresponding compounds and, in the case of aryl amines, are often the base peak in the EI mass spectrum. Figure 5 shows a high-speed SIM mode GC-MS method applied to the analysis of EU-listed azo dye aryl amines.
Figure 5: A high-speed, SIM mode GC-MS method using an Rxi-35Sil MS column and split injection is an excellent technique for routine analysis of the EU-listed azo dye aryl amines in textile extracts.
| Peaks | tR (min) | Quant. Ion (m/z) | Qual. Ion #1 (m/z) | Qual. Ion #2 (m/z) | |
|---|---|---|---|---|---|
| 1. | o-Toluidine | 1.14 | 106 | 107 | 89 |
| 2. | o-Anisidine | 1.56 | 108 | 123 | 80 |
| 3. | 4-Chloroaniline | 1.71 | 127 | 129 | 100 |
| 4. | p-Cresidine | 1.95 | 122 | 137 | 94 |
| 5. | 2,4,5-Trimethylaniline | 1.97 | 120 | 135 | 134 |
| 6. | 3-Chloro-o-toluidine | 2.08 | 141 | 106 | 140 |
| 7. | 4-Chloro-o-toluidine | 2.13 | 141 | 106 | 140 |
| 8. | 2,4-Diaminotoluene | 2.70 | 121 | 122 | 94 |
| 9. | 2,4,5-Trichloroaniline (IS) | 3.25 | 195 | 197 | 199 |
| 10. | 2-Naphthylamine | 3.46 | 143 | 115 | 116 |
| 11. | 2-Aminobiphenyl | 3.50 | 169 | 168 | 167 |
| 12. | 2-Amino-4-nitrotoluene | 3.73 | 152 | 106 | 79 |
| Peaks | tR (min) | Quant. Ion (m/z) | Qual. Ion #1 (m/z) | Qual. Ion #2 (m/z) | |
|---|---|---|---|---|---|
| 13. | 4-Aminobiphenyl | 4.31 | 169 | 168 | 167 |
| 14. | p-Aminoazobenzene | 5.66 | 92 | 197 | 120 |
| 15. | 4,4'-Oxydianiline | 5.90 | 200 | 171 | 108 |
| 16. | 4,4'-Diaminodiphenylmethane | 5.94 | 198 | 197 | 180 |
| 17. | Benzidine | 5.96 | 184 | 185 | 92 |
| 18. | o-Aminoazotoluene | 6.23 | 106 | 225 | 134 |
| 19. | 3,3'-Dimethyl-4,4'-diaminodiphenylmethane | 6.48 | 226 | 120 | 211 |
| 20. | 3,3'-Dimethylbenzidine | 6.57 | 212 | 213 | 106 |
| 21. | 4,4’-Thiodianiline | 6.92 | 216 | 184 | 183 |
| 22. | 3,3'-Dichlorobenzidine | 7.05 | 252 | 254 | 256 |
| 23. | 4,4'-Methylenebis(2-chloroaniline) | 7.05 | 231 | 266 | 195 |
| 24. | 3,3'-Dimethoxybenzidine | 7.09 | 244 | 201 | 186 |
| Column | Rxi-35Sil MS, 15 m, 0.25 mm ID, 0.25 µm (cat.# 13820) |
|---|---|
| Standard/Sample | AccuStandard carcinogenic aryl amine mix (AE-000-49-R1) |
| Diluent: | Ethyl acetate |
| Conc.: | 10 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 10:1) |
| Liner: | Premium 4 mm Precision liner w/wool (cat.# 23305) |
| Inj. Temp.: | 275 °C |
| Oven | |
| Oven Temp.: | 100 °C to 320 °C at 27 °C/min (hold 1.75 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 2.0 mL/min |
| Detector | MS | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mode: | SIM | ||||||||||||||||||||||||||||||||
| SIM Program: | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Transfer Line Temp.: | 330 °C | ||||||||||||||||||||||||||||||||
| Analyzer Type: | Quadrupole | ||||||||||||||||||||||||||||||||
| Source Type: | Inert | ||||||||||||||||||||||||||||||||
| Drawout Plate: | 3 mm ID | ||||||||||||||||||||||||||||||||
| Source Temp.: | 250 °C | ||||||||||||||||||||||||||||||||
| Quad Temp.: | 180 °C | ||||||||||||||||||||||||||||||||
| Solvent Delay Time: | 1.0 min | ||||||||||||||||||||||||||||||||
| Tune Type: | PFTBA | ||||||||||||||||||||||||||||||||
| Ionization Mode: | EI | ||||||||||||||||||||||||||||||||
| Instrument | Agilent 7890A GC & 5975C MSD | ||||||||||||||||||||||||||||||||
Conclusions
Using a high-speed GC-MS method with an Rxi-35Sil MS column is a powerful technique for the analysis of EU-listed azo dye aryl amines in textile extracts. This high-performance GC column offers the selectivity, inertness, and efficiency necessary to provide consistent identification and quantification of aryl amines well below the REACH and OEKO-TEX Standard 100 maximum residue limits. The high efficiency and well-matched stationary phase selectivity allow resolution of positional isomers, and additional aryl amines can be added to an analysis with confidence. Excellent inertness gives good peak shape and response even with small amounts of aryl amines on column. These qualities make the Rxi-35Sil MS the premier GC column for high-throughput routine analysis of azo dye aryl amines.

