Meet Requirements of EPA Method 537.1 PFAS Analysis with Contaminant-Free Workflow
Abstract
The ubiquitous nature of PFAS in the environment makes ensuring a contaminant-free workflow essential. In this application note, we demonstrate that Resprep S-DVB SPE cartridges and related sample preparation products are consistently free of background interferences. In addition, a PFAS delay column effectively removes any contamination that may be present in the instrument. Using the materials and procedure presented here, EPA Method 537.1 requirements for cleanliness, accuracy, and precision were reliably met.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are being analyzed more now than ever before due to growing concern about human exposure and potential adverse health effects. Their persistent nature and widespread use across many industries and in diverse products, ranging from non-stick kitchenware, weatherproof clothes, and aqueous film-forming foam (AFFF) to food packaging coatings, has made them essentially ubiquitous contaminants worldwide. Accordingly, testing of soils, wastewater, drinking water, and other environmental matrices, along with PFAS testing in foods, continues to increase.
In 2020, the U.S. EPA released Method 537.1 revision 2 [1] for PFAS analysis in drinking water. The method specifies that sample preparation must be performed using styrene-divinylbenzene (S-DVB) SPE cartridges and that deviation in the extraction procedure is not allowed. Because background PFAS contaminants can leach out of materials anywhere in the sample pathway and interfere with target compound analysis, labs must demonstrate acceptable background, accuracy, and precision results each time a new lot of supplies is used. In addition to this initial qualification, routine blank and QC sample analysis is required to ensure continued performance.
In a typical workflow, the sample comes in contact with multiple surfaces and substances, each of which can be possible sources of PFAS contamination or retention. These include collection vessels, chemicals (Trizma base, solvents, mobile phases, etc.), pipettes, SPE products, manifolds or automated systems, tubing, filters, vials, vial caps, and components within the LC. Even when care is taken to avoid materials known to leach PFAS, such as PTFE, or to which target analytes may adhere, such as glass, the use of clean, high-quality consumables will help prevent downtime due to system suitability failures.
Here, we followed U.S. EPA Method 537.1 and demonstrated a contaminant-free workflow that meets the stringent method requirements. While this data set is based on Method 537.1, similar testing to determine if background PFAS are present is strongly recommended for other PFAS methods [2] due to the pervasiveness of PFAS contamination.
Experimental
Calibration Standards and Quality Control Samples
PFAS analytical, internal, and surrogate standards were used to create calibration standards and laboratory fortified blanks as directed by EPA 537.1. Eight calibration standards were created from 0.2–50 ppb corresponding to 0.8–200 ppt in drinking water prior to 250-fold concentration, which occurs during sample preparation. Laboratory reagent blanks (LRB) and laboratory fortified blanks (LFB) were used as per EPA 537.1, sections 9.2.2–9.2.4. LFBs spiked at 40 ppt were used to determine the accuracy and precision of the method.
Sample Preparation
Analytical and surrogate standards were added to 250 mL 18.3 MΩ•cm (megohm) ultrapure reagent water for the LFB samples. The LFB samples were kept in polypropylene containers prior to extraction. The materials used for this workflow are detailed in Table I.
PFAS were extracted by using Resprep S-DVB SPE cartridges (6 mL, 500 mg) attached to a Resprep vacuum manifold. Cartridges were first conditioned using 15 mL methanol followed by 18 mL reagent water, never allowing the bed to dry. Reservoirs were affixed to the SPE cartridges with adaptors to avoid the use of PTFE transfer lines. Use of reservoirs, as set up in Figure 1, made the addition of the samples more convenient. The full sample preparation procedure is described below and in Figure 2.
For extraction, a flow rate of approximately 10–15 mL/min was established, and care was taken to never allow the particle bed to dry throughout the extraction process. After the samples had passed through the cartridges, we rinsed each sample bottle with two 7.5 mL aliquots of reagent water. After rinsing the sample bottles, the aliquot was used to rinse each sample reservoir as well to ensure that no PFAS of interest in the sample were left behind.
Following extraction, we dried the SPE cartridges by drawing air through them while they were still attached to the vacuum manifold. After drying, collection tubes were placed in the manifold and two 4 mL aliquots of methanol were passed through each SPE cartridge and collected. The collection tubes were removed from the manifold, and the extract was concentrated to dryness under nitrogen flow while heating the samples at 65 °C.
Once the samples were dry, we added 1 mL 96:4 methanol:water solution and internal standard and then vortexed to ensure proper mixing. After vortexing, aliquots of the concentrated solution were transferred to polypropylene sample vials and capped with polyethylene caps. Samples were then analyzed via an LC-MS/MS equipped with a PFAS delay column (cat.# 27854) and a Raptor C18 LC column (50 mm x 2.1 mm, 2.7 µm; cat.# 9304A52). Method conditions can be found in Figures 3 and 4.
Figure 1: Sample preparation setup using sample reservoirs attached to Resprep S-DVB SPE cartridges mounted on a vacuum manifold.
Figure 2: Sample preparation following Method 537.1. Caution: do not allow cartridge bed to dry during any step.
Table I: Sample preparation materials used for Method 537.1 PFAS analysis.
|
Description |
Restek Cat.# |
|
Resprep S-DVB SPE cartridges (6 mL, 500 mg) |
|
|
Resprep vacuum manifold (12 or 24 port) |
|
|
Reservoirs (polypropylene) |
|
|
Connectors (polypropylene) |
|
|
Vials (polypropylene) |
|
|
Vial caps (polyethylene) |
*A 12-port manifold was used in this study, but either manifold can be employed because the SPE cartridges do not contact the manifold directly; they only contact the quick-replace disposable liners that are used for both manifold styles.
Results and Discussion
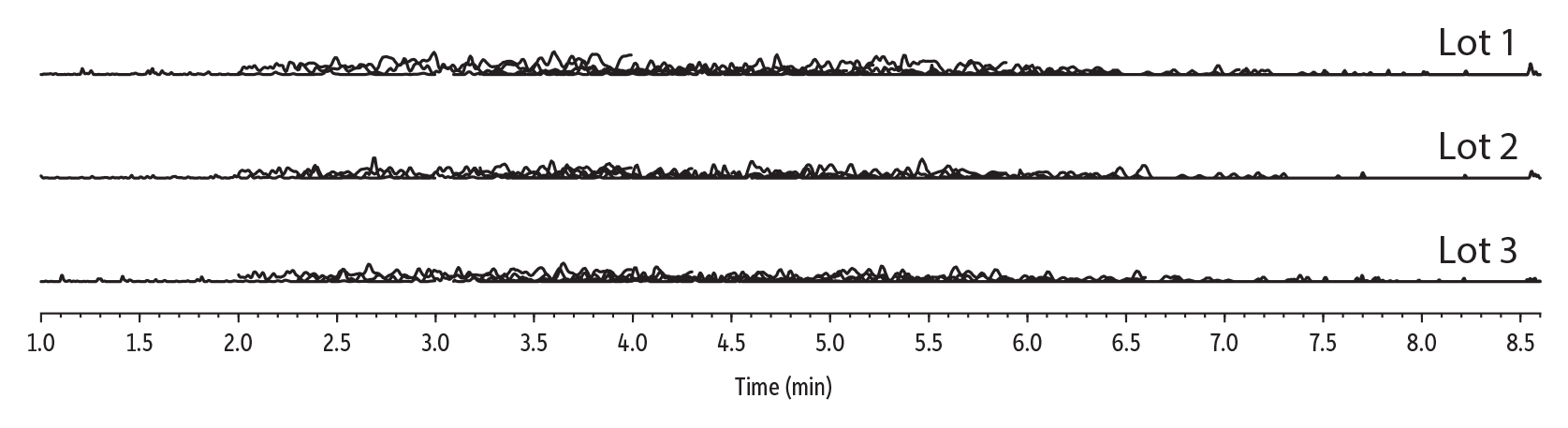
Method 537.1 PFAS analysis in drinking water requirements include initial and ongoing demonstration of low system background and suitable accuracy and precision to ensure that the workflow, from sample collection through analysis, is free of contamination and qualified for use. To verify cleanliness, laboratory reagent blanks were prepared for three different lots of Resprep S-DVB SPE cartridges according to the method. As shown in Figure 3, all lots were free of contamination and no target analytes were detected, satisfying the low system background requirement of section 9.2.2. LOD values were 0.2–5 ppt, defined as a signal-to-noise ratio >3 for each compound. LOQ values were established as signal-to-noise ratios >10 and were found to be 0.5–10 ppt across the range of target analytes.
In addition to demonstrating the consistent cleanliness of Resprep S-DVB cartridges across multiple lots, this experiment proved that no interfering contaminants leached from any other component in the entire sample prep workflow listed in Table I (vacuum manifold system, vials, and caps, etc.). LC instruments can also contribute background contamination, but none was present in these analyses because the LC was plumbed with PEEK or stainless-steel tubing, and a PFAS delay column was installed. A PFAS delay column prevents interference from any PFAS leaching out of components in the LC system by trapping them and delaying their elution until after the sample analytes have eluted. Retention on a PFAS delay column is strong enough to prevent breakthrough even with extended equilibration times. [3,4]
Figure 3: Multi-Lot Laboratory Reagent Blanks (LRB)
| Column | Raptor C18 (cat.# 9304A52) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 96:4 Methanol:water | ||||||||||||||||||||
| Conc.: | 0 ng/mL laboratory reagent blank | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | HPLC |
| Sample Preparation | The sample was prepared using Resprep S-DVB SPE cartridges (cat.# 28937) mounted on a Resprep vacuum manifold (cat.# 29298-VM) following the procedure in U.S. EPA Method 537.1. |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
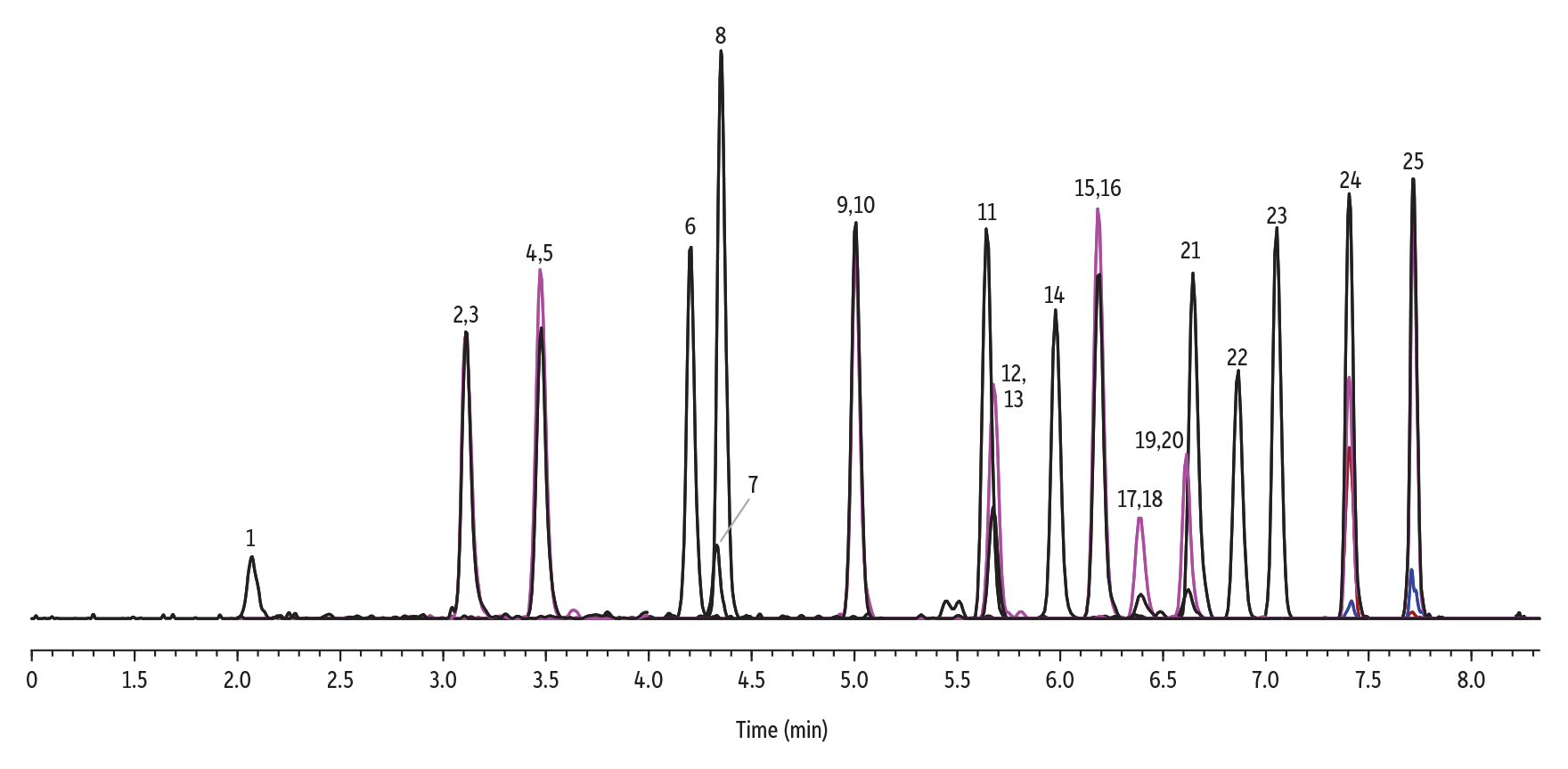
Recovery performance for Method 537.1 PFAS analysis was assessed using four laboratory fortified blanks prepared at 40 ppt. A representative LFB chromatogram is presented in Figure 4, which shows that good peak efficiency, selectivity, and asymmetry were obtained. To meet the method requirements for recovery, precision values across the LFB replicates must have %RSD <20%, and accuracy results for the same LFB samples must be within ±30% of the true value. The data presented in Table II demonstrate that method precision and accuracy (Section 9.2.3. and Section 9.2.4. of Method 537.1, respectively) requirements were easily met. Good recoveries indicate that the target analytes were not lost due to adhesion to surfaces encountered in the sample pathway.
Figure 4: Laboratory Fortified Blank (LFB) at Midrange (40 ppt)
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 1. | Perfluorobutanesulfonic acid (PFBS) | 2.08 | 40 | 299.0 | 80.0 |
| 2. | Perfluoro-n-[1,2-13C2]hexanoic acid (13C2-PFHxA) | 3.11 | 20 | 315.1 | 270.1 |
| 3. | Perfluorohexanoic acid (PFHxA) | 3.11 | 40 | 313.2 | 269.0 |
| 4. | Tetrafluoro-2-heptafluoropropoxy-13C3-propanoic acid (13C3-HFPO-DA) | 3.47 | 20 | 332.1 | 287.3 |
| 5. | Hexafluoropropylene oxide dimer acid (HFPO-DA) | 3.47 | 40 | 328.9 | 284.9 |
| 6. | Perfluoroheptanoic acid (PFHpA) | 4.19 | 40 | 363.2 | 319.2 |
| 7. | Perfluorohexanesulfonic acid (PFHxS) | 4.33 | 40 | 399.2 | 79.9 |
| 8. | 4,8-Dioxa-3H-perfluorononanoic acid (ADONA) | 4.34 | 40 | 376.9 | 251.0 |
| 9. | Perfluoro-[1,2-13C2]octanoic acid (13C2-PFOA) | 5.00 | 20 | 414.9 | 370.0 |
| 10. | Perfluorooctanoic acid (PFOA) | 5.00 | 40 | 413.1 | 369.1 |
| 11. | Perfluorononanoic acid (PFNA) | 5.64 | 40 | 463.1 | 419.0 |
| 12. | Perfluorooctanesulfonic acid (PFOS) | 5.66 | 40 | 499.2 | 80.1 |
| Peaks | tR (min) | Conc. (ng/L) | Precursor Ion | Product Ion | |
|---|---|---|---|---|---|
| 13. | Perfluoro-1-[1,2,3,4-13C4]octanesulfonic acid (13C4-PFOS) | 5.67 | 60 | 503.1 | 80.2 |
| 14. | 9-Chlorohexadecafluoro-3-oxanone-1-sulfonic acid (9Cl-PF3ONS) | 5.97 | 40 | 531.0 | 350.9 |
| 15. | Perfluoro-n-[1,2-13C2]decanoic acid (13C2-PFDA) | 6.18 | 20 | 515.2 | 470.1 |
| 16. | Perfluorodecanoic acid (PFDA) | 6.18 | 40 | 512.9 | 468.9 |
| 17. | N-methyl perfluorooctanesulfonamidoacetic acid (N-MeFOSAA) | 6.38 | 40 | 570.2 | 419.0 |
| 18. | N-deuteriomethylperfluoro-1-octanesulfonamidoacetic acid (d3-N-MeFOSAA) | 6.38 | 80 | 573.1 | 419.1 |
| 19. | N-deuterioethylperfluoro-1-octanesulfonamidoacetic acid (d5-N-EtFOSAA) | 6.61 | 80 | 589.2 | 419.1 |
| 20. | N-ethyl perfluorooctanesulfonamidoacetic acid (N-EtFOSAA) | 6.62 | 40 | 583.8 | 418.9 |
| 21. | Perfluoroundecanoic acid (PFUnA) | 6.64 | 40 | 563.2 | 519.1 |
| 22. | 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) | 6.86 | 40 | 630.8 | 451.1 |
| 23. | Perfluorododecanoic acid (PFDoA) | 7.05 | 40 | 613.1 | 569.1 |
| 24. | Perfluorotridecanoic acid (PFTrDA) | 7.40 | 40 | 663.0 | 619.2 |
| 25. | Perfluorotetradecanoic acid (PFTA) | 7.71 | 40 | 713.1 | 669.0 |
| Column | Raptor C18 (cat.# 9304A52) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 50 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | 96:4 Methanol:water | ||||||||||||||||||||
| Conc.: | 5-20 ng/mL in the final solution after sample preparation (equivalent to 20-80 ppt in laboratory reagent water sample prior to extraction) | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | Water, 5 mM ammonium acetate | ||||||||||||||||||||
| B: | Methanol | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI- |
| Mode: | MRM |
| Instrument | HPLC |
| Sample Preparation | The sample was prepared using Resprep S-DVB SPE cartridges (cat.# 28937) mounted on a Resprep vacuum manifold (cat.# 29298-VM) following the procedure in U.S. EPA Method 537.1. While internal standard concentrations varied, all target analytes were fortified at 40 ppt. |
| Notes | A PFAS delay column (cat.# 27854) was installed before the injector. |
Table II: Precision and Accuracy Results for Method 537.1 PFAS Analysis of 40 ppt Laboratory Fortified Blanks (n = 4).
|
Analyte |
%RSD* |
Mean Recovery** |
|
Perfluorobutanesulfonic acid (PFBS) |
11.9% |
91.1% |
|
Perfluorohexanoic acid (PFHxA) |
7.96% |
99.4% |
|
Hexafluoropropylene oxide dimer acid (HFPO-DA) |
6.34% |
94.4% |
|
Perfluoroheptanoic acid (PFHpA) |
4.19% |
92.7% |
|
Perfluorohexanesulfonic acid (PFHxS) |
11.9% |
89.4% |
|
4,8-Dioxa-3H-perfluorononanoic acid (ADONA) |
5.18% |
96.6% |
|
Perfluorooctanoic acid (PFOA) |
5.21% |
91.6% |
|
Perfluorononanoic acid (PFNA) |
6.79% |
97.2% |
|
Perfluorooctanesulfonic acid (PFOS) |
6.78% |
87.8% |
|
9-Chlorohexadecafluoro-3-oxanone-1-sulfonic acid (9Cl-PF3ONS) |
8.59% |
85.1% |
|
Perfluorodecanoic acid (PFDA) |
6.96% |
93.6% |
|
N-methyl perfluorooctanesulfonamidoacetic acid (N-MeFOSAA) |
10.1% |
82.8% |
|
N-ethyl perfluorooctanesulfonamidoacetic acid (N-EtFOSAA) |
16.5% |
106% |
|
Perfluoroundecanoic acid (PFUnA) |
2.30% |
97.5% |
|
11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS) |
5.47% |
87.6% |
|
Perfluorododecanoic acid (PFDoA) |
5.73% |
99.0% |
|
Perfluorotridecanoic acid (PFTrDA) |
12.7% |
89.1% |
|
Perfluorotetradecanoic acid (PFTA) |
8.90% |
89.7% |
*%RSD must be <20%.
**Recovery must within ±30% of the true value.
Conclusion
The data presented here clearly demonstrate that Resprep S-DVB SPE cartridges and the other sample preparation products used in this workflow for EPA Method 537.1 PFAS analysis were consistently free of background contaminants. In addition, use of a PFAS delay column effectively removed any PFAS background contamination that was potentially present in the LC instrument. Based on the results shown here, use of these workflow consumables will reduce interfering background contamination, leading to reliable system qualification and more accurate analysis and reporting.
References
- J. Shoemaker and D. Tettenhorst, U.S. EPA Method 537.1 Rev 2., Method 537.1 Determination of selected per- and polyflourinated alkyl substances in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS), 2020. https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=539984&Lab=CESER
- Restek Corporation, Product guide for PFAS analysis: a methods-based reference to lab supplies for PFAS testing (EVAR3498-UNV), (2021). https://www.restek.com/articles/product-guide-for-pfas-analysis
- Restek Corporation, Eliminate the impact of instrument-related PFAS interferences by using a delay column, (2019). https://www.restek.com/articles/eliminate-the-impact-of-instrument-related-pfas-interferences-by-using-a-delay-column
- Restek, PFAS Analysis – Why a Delay Column is Important, Video. https://www.restek.com/videos/pfas-analysis-why-a-delay-column-is-important

