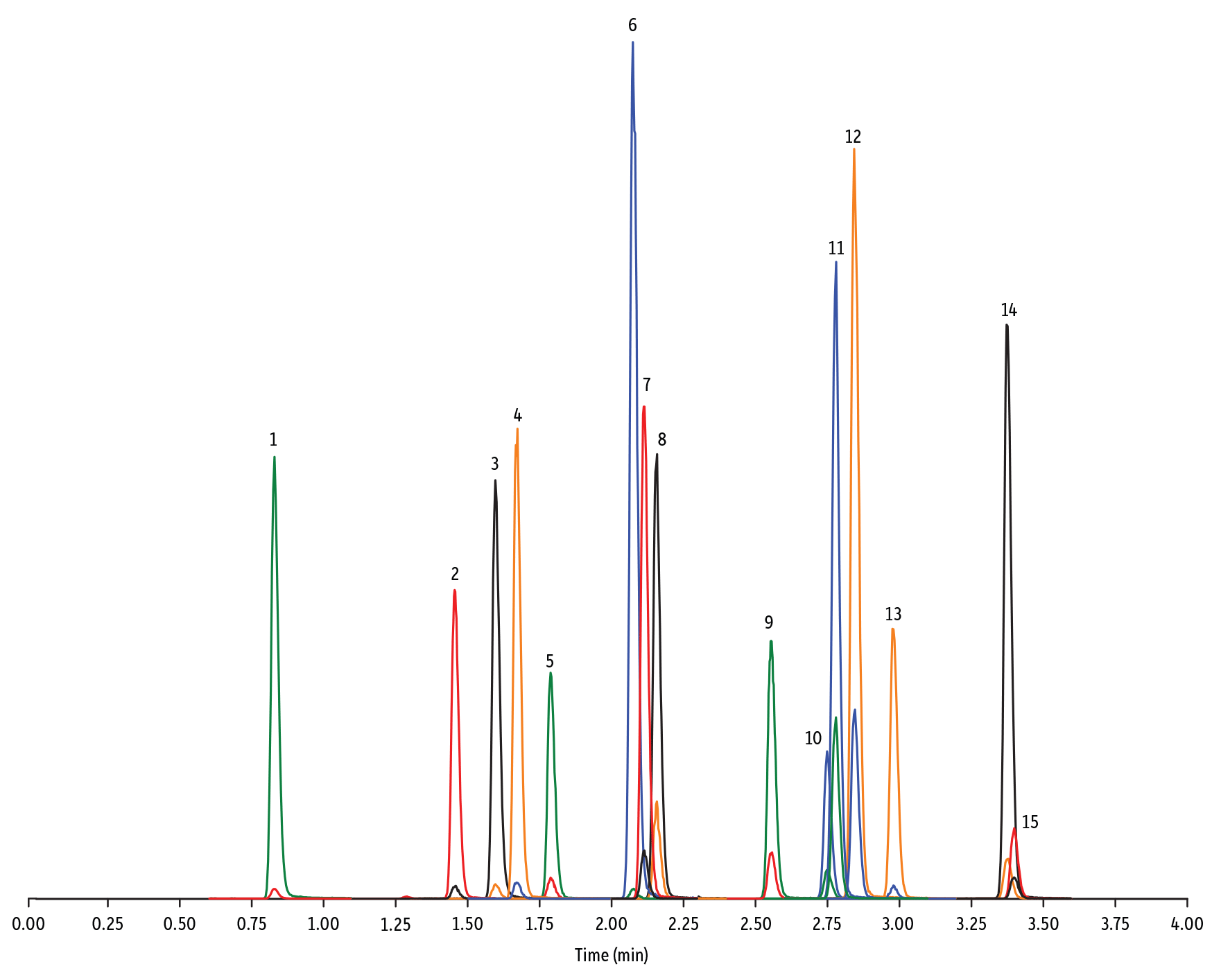
One Analysis, One Column, Less than 9 Minutes for Over 60 Multiclass Antibiotics
Featured Application: Multiclass Veterinary Antibiotics on Raptor C18 by LC-MS/MS
- Highly efficient peak separation and fast analysis times.
- Versatility and global applicability for antibiotic residue analysis—capable of individual class panel optimization for quantitation:
- Macrolide, Lincosamide, and Streptogramin (Figure 1)
- Amphenicol and Tetracycline (Figure 2)
- Quinolone (Figure 3)
- Penicillin, Cephalosporin, and Tetracycline (Figure 4)
- Sulfonamide (Figure 5) (For Ionophore, use on Raptor Biphenyl. [Figure 6])
The use of antibiotics on food-producing animals is a public health and safety concern due to the potential of generating drug-resistant bacteria. Many countries in the European Union and Canada have banned the use of antibiotics for nontherapeutic purposes, and the United States is implementing a policy to reduce the use of medically important antibiotics for growth promotion. To regulate the proper use of veterinary antibiotics, the U.S. FDA has set maximum residue limits (MRL) for a variety of animal tissue and food products (21 CFR Part 556). A sensitive, efficient, and reliable analytical method for different classes of antibiotics is necessary to meet this regulation, and the Raptor C18 LC column is the ideal choice.
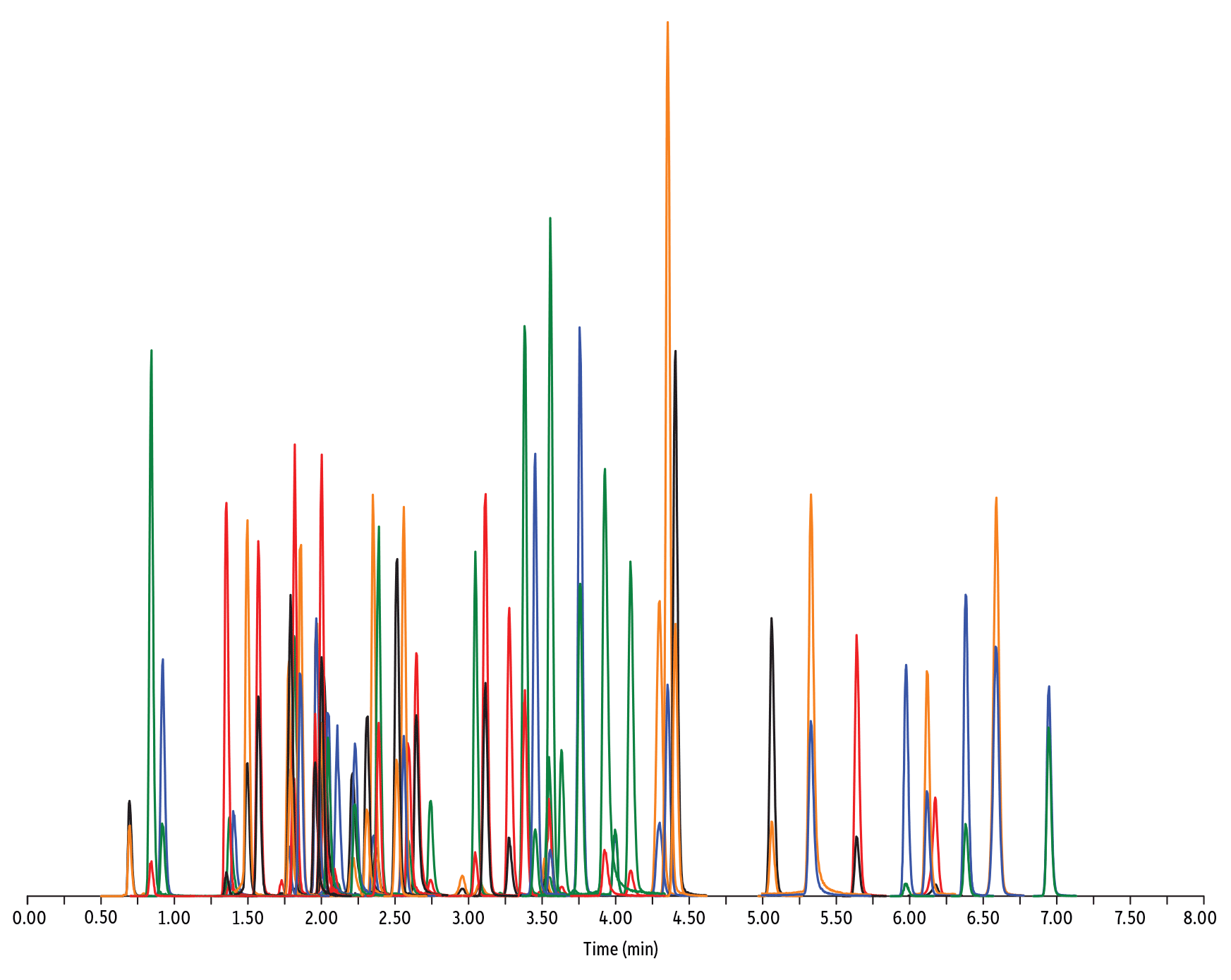
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Desacetyl cephapirin | 0.70 | 150 | 382.03 | 111.92 | 124.21 |
| 2. | Sulfanilamide | 0.85 | 200 | 172.98 | 93.07 | 75.23 |
| 3. | Amoxicillin | 0.92 | 100 | 366.24 | 349.10 | 208.07 |
| 4. | Cephapirin | 1.36 | 50 | 424.17 | 292.08 | 124.14 |
| 5. | Tildipirosin | 1.38 | 200 | 734.59 | 561.45 | 204.15 |
| 6. | Desfuroyl ceftiofur cysteine disulfide | 1.40 | 300 | 549.16 | 183.02 | 126.00 |
| 7. | Lincomycin | 1.50 | 50 | 407.32 | 359.23 | 389.28 |
| 8. | Sulfadiazine | 1.57 | 20 | 251.18 | 156.04 | 92.08 |
| 9. | Cefquinome | 1.73 | 200 | 529.19 | 134.10 | 125.12 |
| 10. | Ampicillin | 1.78 | 50 | 350.19 | 106.07 | 160.06 |
| 11. | Sulfathiazole | 1.79 | 10 | 256.16 | 156.03 | 92.08 |
| 12. | Marbofloxacin | 1.81 | 10 | 363.20 | 72.11 | 320.10 |
| 13. | Cefalexin | 1.82 | 100 | 348.10 | 158.05 | 174.05 |
| 14. | Sulfapyridine | 1.86 | 10 | 250.13 | 156.10 | 92.08 |
| 15. | Norfloxacin | 1.96 | 20 | 320.23 | 276.20 | 233.13 |
| 16. | Ofloxacin | 1.98 | 10 | 362.21 | 318.20 | 261.15 |
| 17. | Sulfamerazine | 2.00 | 20 | 265.08 | 156.03 | 92.08 |
| 18. | Cefalonium | 2.01 | 100 | 459.16 | 337.03 | 123.10 |
| 19. | Oxytetracycline | 2.02 | 25 | 461.27 | 426.15 | 443.32 |
| 20. | Ciprofloxacin | 2.04 | 20 | 332.18 | 288.22 | 245.15 |
| 21. | Cefacetrile | 2.09 | 300 | 362.07 | 258.08 | 178.01 |
| 22. | Tulathromycin A | 2.11 | 100 | 806.65 | 577.42 | 420.31 |
| 23. | Tetracycline | 2.21 | 25 | 445.28 | 154.07 | 427.32 |
| 24. | Danofloxacin | 2.23 | 20 | 358.22 | 340.16 | 314.21 |
| 25. | Enrofloxacin | 2.32 | 10 | 360.29 | 316.22 | 245.13 |
| 26. | Orbifloxacin | 2.35 | 10 | 396.22 | 352.17 | 226.12 |
| 27. | Thiamphenicol* | 2.38 | 200 | 354.16 | 290.04 | 184.98 |
| 28. | Sulfamethazine | 2.39 | 10 | 279.23 | 186.08 | 124.08 |
| 29. | Sulfamethizole | 2.52 | 10 | 271.17 | 156.02 | 108.02 |
| 30. | Sulfamethoxypyridazine | 2.56 | 10 | 281.14 | 156.03 | 126.07 |
| 31. | Sarafloxacin | 2.59 | 10 | 386.20 | 342.20 | 368.15 |
| 32. | Difloxacin | 2.65 | 10 | 400.23 | 356.17 | 299.13 |
| 33. | Cefazolin | 2.75 | 100 | 455.10 | 323.06 | 295.09 |
| 34. | Spiramycin | 2.96 | 200 | 843.64 | 540.36 | 699.48 |
| 35. | Pirlimycin | 3.05 | 20 | 411.32 | 363.18 | 327.21 |
| 36. | Chlortetracycline | 3.08 | 25 | 479.27 | 154.07 | 371.06 |
| 37. | Sulfachlorpyridazine | 3.12 | 20 | 285.05 | 156.03 | 108.09 |
| 38. | Gamithromycin | 3.28 | 100 | 777.63 | 619.52 | 601.45 |
| 39. | Sulfadoxine | 3.39 | 10 | 311.17 | 156.03 | 108.09 |
| 40. | Sulfamethoxazole | 3.46 | 20 | 254.18 | 155.98 | 147.06 |
| 41. | Cefoperazone | 3.52 | 100 | 646.26 | 143.07 | 148.02 |
| 42. | Florfenicol* | 3.55 | 200 | 356.10 | 336.02 | 184.98 |
| 43. | Sulfaethoxypyridazine | 3.56 | 20 | 295.17 | 267.07 | 156.03 |
| 44. | Tilmicosin | 3.64 | 100 | 869.72 | 696.50 | 522.42 |
| 45. | Sulfisoxazole | 3.76 | 20 | 268.14 | 156.03 | 113.10 |
| 46. | Oxolinic acid | 3.94 | 5 | 262.10 | 244.06 | 215.96 |
| 47. | Chloramphenicol* | 4.00 | 200 | 321.16 | 151.99 | 257.04 |
| 48. | Ceftiofur | 4.11 | 50 | 524.14 | 241.08 | 125.24 |
| 49. | Erythromycin | 4.31 | 25 | 734.64 | 576.40 | 558.38 |
| 50. | Sulfadimethoxine | 4.36 | 10 | 311.17 | 156.09 | 108.09 |
| 51. | Sulfaquinoxaline | 4.42 | 20 | 301.18 | 156.04 | 108.02 |
| 52. | Tylosin** | 4.67 | 100 | 916.62 | 772.49 | 598.36 |
| 53. | Penicillin G | 5.07 | 100 | 335.18 | 176.07 | 160.07 |
| 54. | Flumequine | 5.34 | 5 | 262.15 | 244.11 | 202.03 |
| 55. | Penicillin V | 5.56 | 100 | 351.10 | 160.06 | 114.07 |
| 56. | Oxacillin | 5.99 | 100 | 402.15 | 160.05 | 114.06 |
| 57. | Virginiamycin M1 | 6.13 | 50 | 526.43 | 508.31 | 355.10 |
| 58. | Tylvalosin | 6.19 | 50 | 1042.71 | 814.46 | 640.39 |
| 59. | Cloxacillin | 6.39 | 100 | 436.15 | 277.06 | 160.05 |
| 60. | Nafcillin | 6.60 | 25 | 415.19 | 199.09 | 171.06 |
| 61. | Dicloxacillin | 6.96 | 100 | 470.11 | 160.05 | 311.02 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||||||
| Temp.: | 35 °C | ||||||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||||||
| Conc.: | 5–300 ng/mL | ||||||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+/ESI- |
| Mode: | Scheduled MRM |
| Instrument | UHPLC |
| Notes | 1. Positive and negative polarity data were collected simultaneously from a single injection. 2. Amphenicol compounds (chloramphenicol, thiamphenicol, and florfenicol) were detected with negative polarity. 3. The MRM was scheduled at +/- 20 to 30 seconds for each analyte. 4. Multiclass antibiotics include penicillin, cephalosporin, tetracycline, sulfonamide, macrolide, lincosamide, streptogramin, amphenicol, and quinolone. **The retention time for Tylosin is noted in the peak list; however, it was not included in the chromatogram. |
Figure 1: Macrolide, Lincosamide, and Streptogramin Antibiotics on Raptor C18 by LC-MS/MS
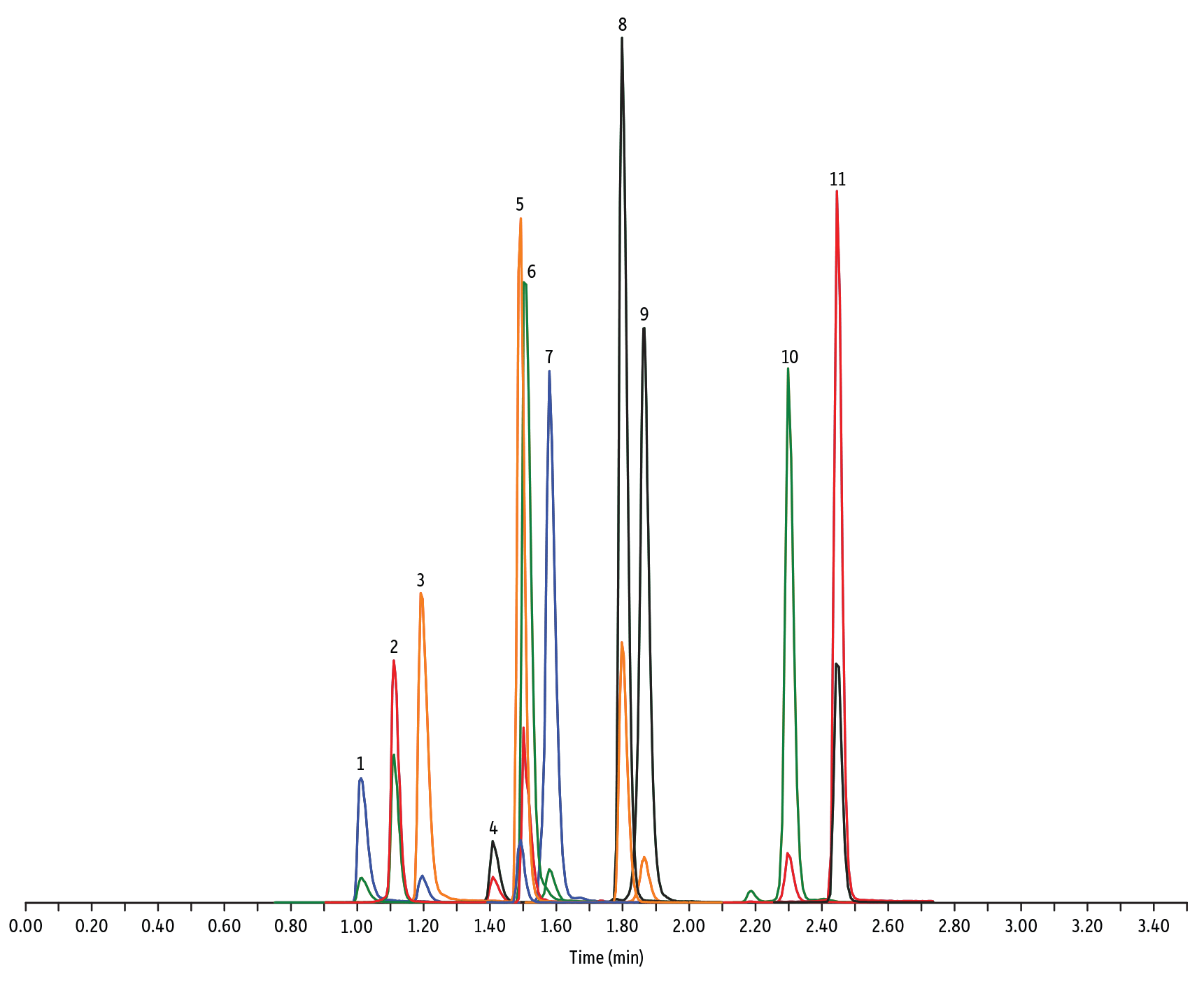
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Tildipirosin | 1.01 | 200 | 734.59 | 561.45 | 204.15 |
| 2. | Lincomycin | 1.11 | 50 | 407.32 | 359.23 | 389.28 |
| 3. | Tulathromycin A | 1.19 | 100 | 806.65 | 577.42 | 420.31 |
| 4. | Spiramycin | 1.41 | 200 | 843.64 | 540.36 | 699.48 |
| 5. | Pirlymycin | 1.49 | 20 | 411.32 | 363.18 | 327.21 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 6. | Gamithromycin | 1.50 | 100 | 777.63 | 619.52 | 601.45 |
| 7. | Tilmicosin | 1.58 | 100 | 869.72 | 696.50 | 522.42 |
| 8. | Erythromycin | 1.80 | 25 | 734.64 | 576.40 | 558.38 |
| 9. | Tylosin | 1.87 | 100 | 916.62 | 772.49 | 598.36 |
| 10. | Tylvalosin | 2.30 | 50 | 1042.71 | 814.46 | 640.39 |
| 11. | Virginiamycin M1 | 2.45 | 50 | 526.43 | 508.31 | 355.10 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 20–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
Figure 2: Amphenicol and Tetracycline Antibiotics on Raptor C18 by LC-MS/MS
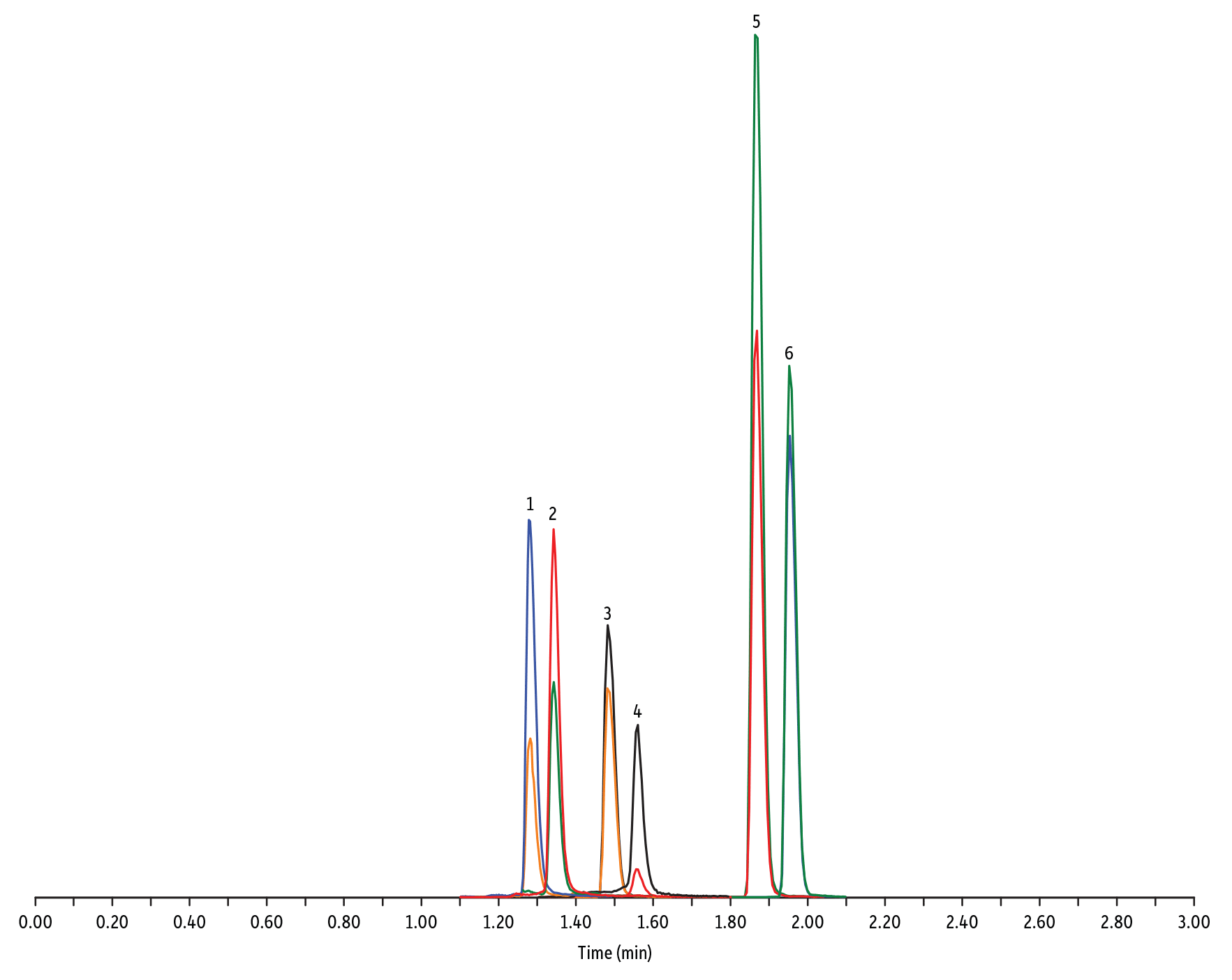
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Oxytetracycline | 1.28 | 25 | 461.27 | 426.15 | 443.32 |
| 2. | Tetracycline | 1.34 | 25 | 445.28 | 154.07 | 427.32 |
| 3. | Thiamphenicol* | 1.48 | 200 | 354.16 | 290.04 | 184.98 |
| 4. | Chlortetracycline | 1.56 | 25 | 479.27 | 154.07 | 371.06 |
| 5. | Florfenicol* | 1.86 | 200 | 356.10 | 336.02 | 184.98 |
| 6. | Chloramphenicol* | 1.95 | 200 | 321.16 | 151.99 | 257.04 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 25–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+/ESI- |
| Mode: | MRM |
| Instrument | UHPLC |
| Notes | Tetracyclines and amphenicols were analyzed with ESI+ and ESI- mode, respectively. |
Figure 3: Quinolone Antibiotics on Raptor C18 by LC-MS/MS
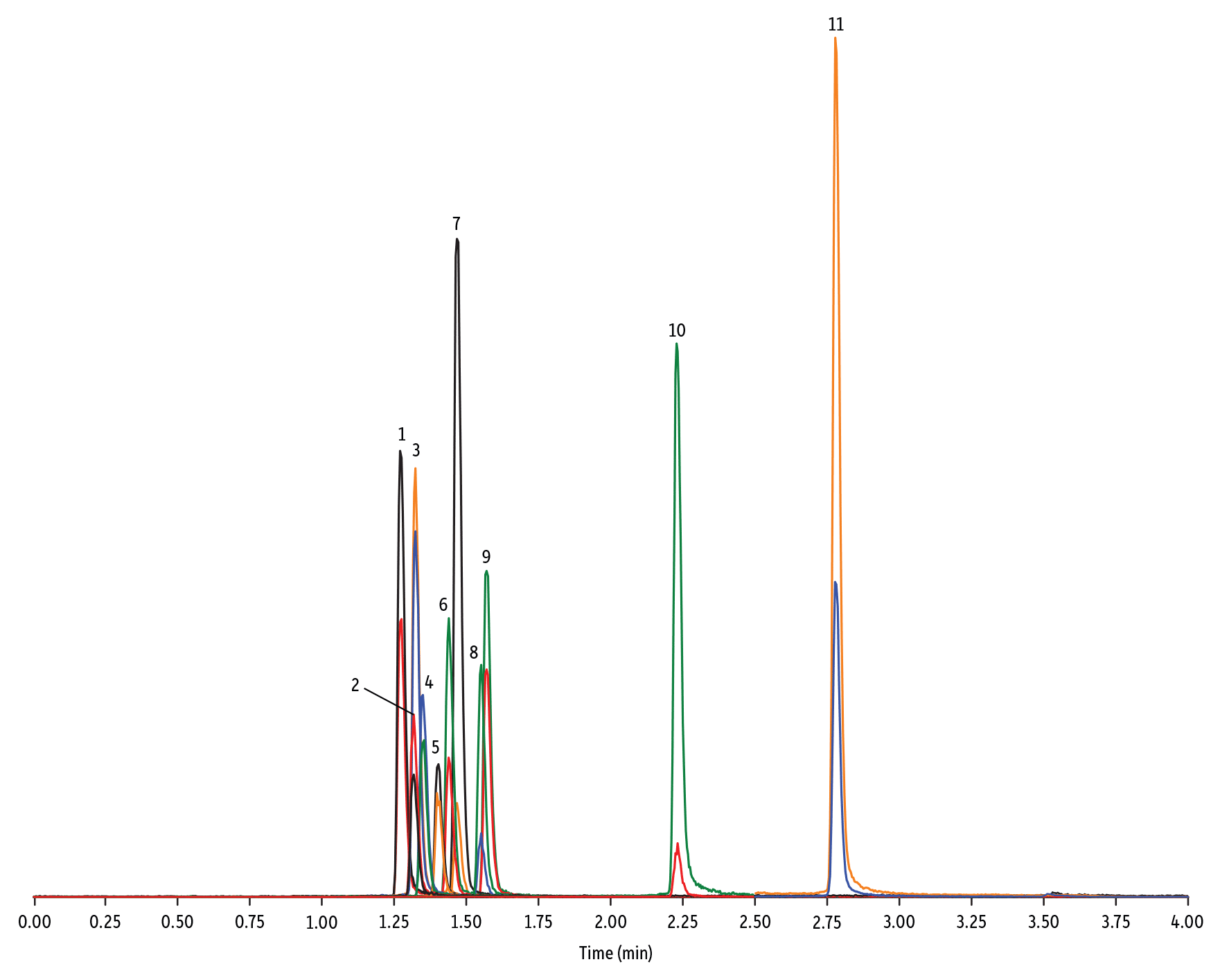
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Marbofloxacin | 1.28 | 10 | 363.20 | 72.11 | 320.10 |
| 2. | Norfloxacin | 1.32 | 20 | 320.23 | 276.20 | 233.13 |
| 3. | Ofloxacin | 1.32 | 10 | 362.21 | 318.20 | 261.15 |
| 4. | Ciprofloxacin | 1.35 | 20 | 332.18 | 288.22 | 245.15 |
| 5. | Danofloxacin | 1.40 | 20 | 358.22 | 340.16 | 314.21 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 6. | Enrofloxacin | 1.44 | 10 | 360.29 | 316.22 | 245.13 |
| 7. | Orbifloxacin | 1.47 | 10 | 396.22 | 352.17 | 226.12 |
| 8. | Sarafloxacin | 1.55 | 10 | 386.20 | 342.20 | 368.15 |
| 9. | Difloxacin | 1.57 | 10 | 400.23 | 356.17 | 299.13 |
| 10. | Oxolinic acid | 2.23 | 5 | 262.10 | 244.06 | 215.96 |
| 11. | Flumequine | 2.78 | 5 | 262.15 | 244.11 | 202.03 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 5–20 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
Figure 4: Penicillin, Cephalosporin, and Tetracycline Antibiotics on Raptor C18 by LC-MS/MS
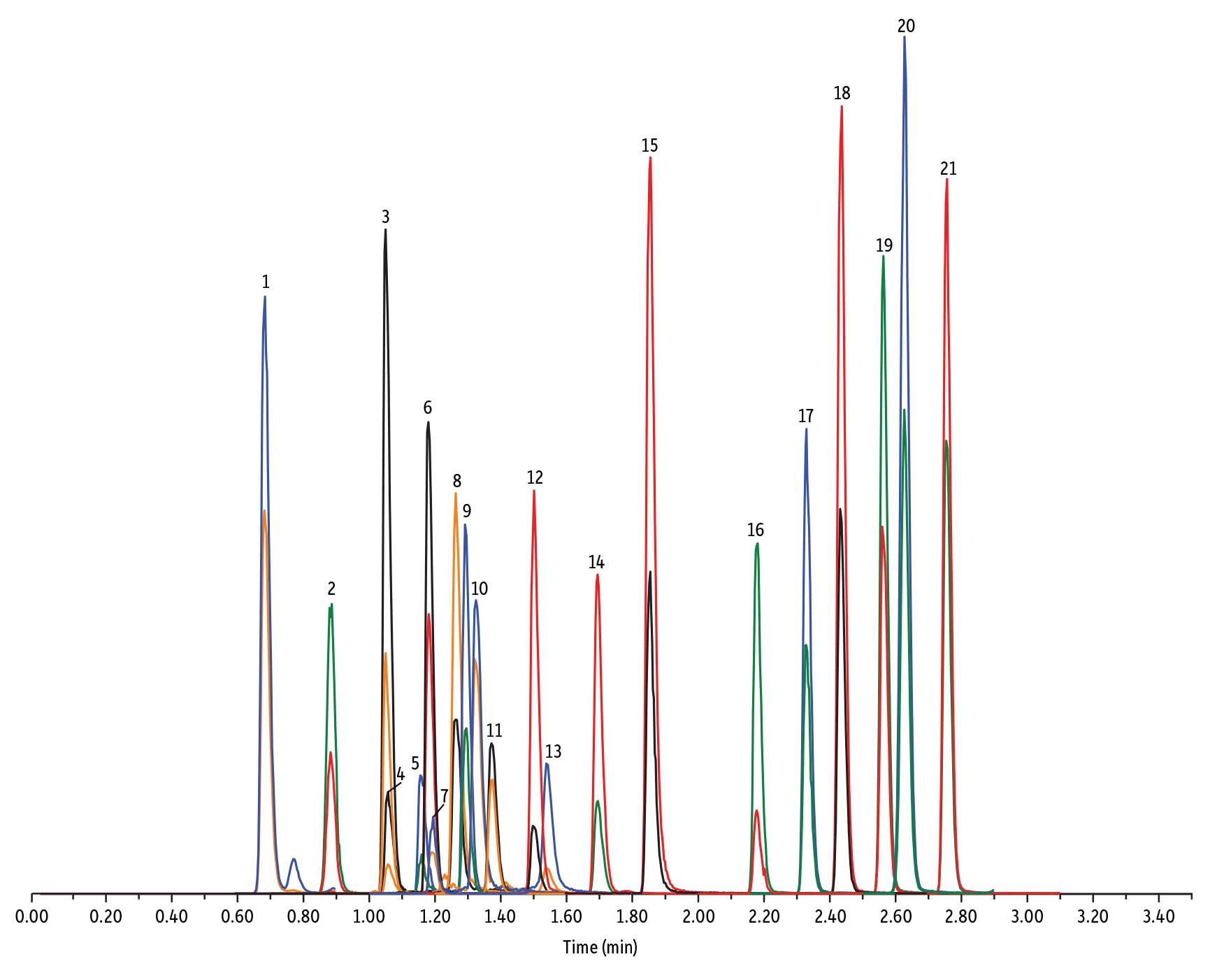
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Desacetyl cephapirin | 0.68 | 150 | 382.03 | 111.92 | 124.21 |
| 2. | Amoxicillin | 0.89 | 100 | 366.24 | 349.10 | 208.07 |
| 3. | Cephapirin | 1.05 | 50 | 424.17 | 292.08 | 124.14 |
| 4. | Desfuroyl ceftiofur cysteine disulfide | 1.06 | 300 | 549.16 | 183.02 | 126.00 |
| 5. | Cefquinome | 1.16 | 200 | 529.19 | 134.10 | 125.12 |
| 6. | Ampicillin | 1.18 | 50 | 350.19 | 106.07 | 160.06 |
| 7. | Cefalexin | 1.19 | 100 | 348.10 | 158.05 | 174.05 |
| 8. | Oxytetracycline | 1.26 | 50 | 461.27 | 426.15 | 443.32 |
| 9. | Cefalonium | 1.29 | 100 | 459.16 | 337.03 | 123.10 |
| 10. | Tetracycline | 1.32 | 50 | 445.28 | 154.07 | 427.32 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 11. | Cefacetrile | 1.37 | 300 | 362.07 | 258.08 | 178.01 |
| 12. | Cefazolin | 1.50 | 100 | 455.10 | 323.06 | 295.09 |
| 13. | Chlortetracycline | 1.54 | 50 | 479.27 | 154.07 | 371.06 |
| 14. | Cefoperazone | 1.69 | 100 | 646.26 | 143.07 | 148.02 |
| 15. | Ceftiofur | 1.85 | 50 | 524.14 | 241.08 | 125.24 |
| 16. | Penicillin G | 2.18 | 100 | 335.18 | 176.07 | 160.07 |
| 17. | Penicillin V | 2.33 | 100 | 351.10 | 160.06 | 114.07 |
| 18. | Oxacillin | 2.44 | 100 | 402.15 | 160.05 | 114.06 |
| 19. | Cloxacillin | 2.56 | 100 | 436.15 | 277.06 | 160.05 |
| 20. | Nafcillin | 2.63 | 25 | 415.19 | 199.09 | 171.06 |
| 21. | Dicloxacillin | 2.76 | 100 | 470.11 | 160.05 | 311.02 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 25–300 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
Figure 5: Sulfonamide Antibiotics on Raptor C18 by LC-MS/MS
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Sulfanilamide | 0.83 | 200 | 172.98 | 93.07 | 75.23 |
| 2. | Sulfadiazine | 1.45 | 20 | 251.18 | 156.04 | 92.08 |
| 3. | Sulfathiazole | 1.60 | 10 | 256.16 | 156.03 | 92.08 |
| 4. | Sulfapyridine | 1.67 | 10 | 250.13 | 156.10 | 92.08 |
| 5. | Sulfamerazine | 1.79 | 20 | 265.08 | 156.03 | 92.08 |
| 6. | Sulfamethazine | 2.07 | 10 | 279.23 | 186.08 | 124.08 |
| 7. | Sulfamethizole | 2.11 | 10 | 271.17 | 156.02 | 108.02 |
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 8. | Sulfamethoxypyridazine | 2.16 | 10 | 281.14 | 156.03 | 126.07 |
| 9. | Sulfachlorpyridazine | 2.55 | 20 | 285.05 | 156.03 | 108.09 |
| 10. | Sulfadoxine | 2.75 | 10 | 311.17 | 156.03 | 108.09 |
| 11. | Sulfamethoxazole | 2.78 | 20 | 254.18 | 155.98 | 147.06 |
| 12. | Sulfaethoxypyridazine | 2.84 | 20 | 295.17 | 267.07 | 156.03 |
| 13. | Sulfisoxazole | 2.98 | 20 | 268.14 | 156.03 | 113.10 |
| 14. | Sulfadimethoxine | 3.37 | 10 | 311.17 | 156.09 | 108.09 |
| 15. | Sulfaquinoxaline | 3.40 | 20 | 301.18 | 156.04 | 108.02 |
| Column | Raptor C18 (cat.# 9304A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor C18 EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9304A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water | ||||||||||||||||||||
| Conc.: | 10–200 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.1% Formic acid in water | ||||||||||||||||||||
| B: | 0.1% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |
Figure 6: Ionophore Antibiotics on Raptor Biphenyl by LC-MS/MS
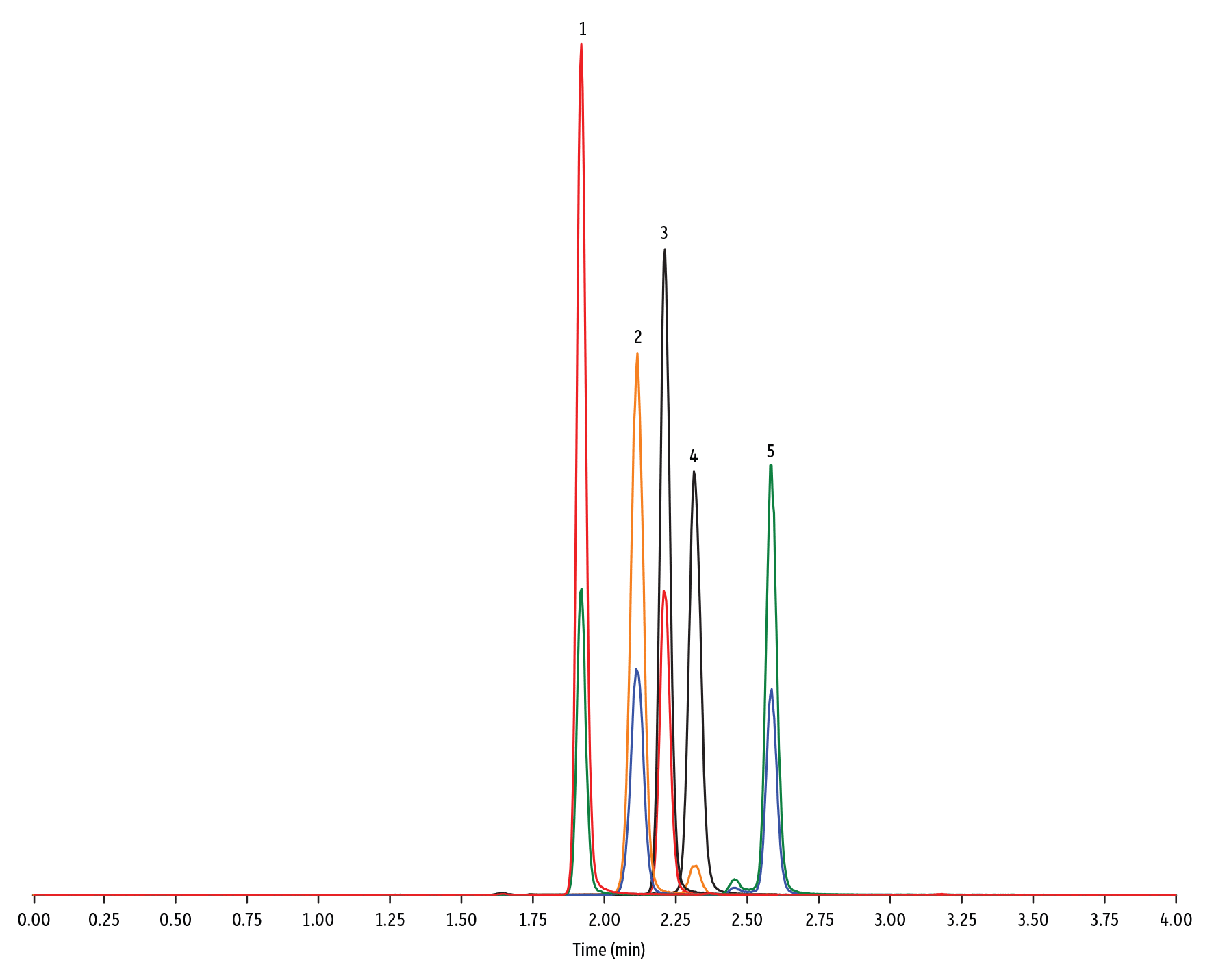
| Peaks | tR (min) | Conc. (ng/mL) | Precursor Ion | Product Ion | Product Ion | |
|---|---|---|---|---|---|---|
| 1. | Lasalocid A | 1.92 | 100 | 613.42 | 377.28 | 595.40 |
| 2. | Monensin | 2.12 | 100 | 693.50 | 675.44 | 461.30 |
| 3. | Salinomycin | 2.19 | 100 | 773.57 | 431.24 | 531.39 |
| 4. | Maduramicin | 2.30 | 100 | 939.65 | 877.58 | 473.31 |
| 5. | Narasin | 2.58 | 100 | 787.59 | 431.27 | 531.35 |
| Column | Raptor Biphenyl (cat.# 9309A12) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimensions: | 100 mm x 2.1 mm ID | ||||||||||||||||||||
| Particle Size: | 2.7 µm | ||||||||||||||||||||
| Pore Size: | 90 Å | ||||||||||||||||||||
| Guard Column: | Raptor Biphenyl EXP guard column cartridge 5 mm, 2.1 mm ID, 2.7 µm (cat.# 9309A0252) | ||||||||||||||||||||
| Temp.: | 40 °C | ||||||||||||||||||||
| Standard/Sample | |||||||||||||||||||||
| Diluent: | Water:methanol (10:90) | ||||||||||||||||||||
| Conc.: | 100 ng/mL | ||||||||||||||||||||
| Inj. Vol.: | 2 µL | ||||||||||||||||||||
| Mobile Phase | |||||||||||||||||||||
| A: | 0.5% Formic acid in water | ||||||||||||||||||||
| B: | 0.5% Formic acid in acetonitrile | ||||||||||||||||||||
|
| Detector | MS/MS |
|---|---|
| Ion Mode: | ESI+ |
| Mode: | MRM |
| Instrument | UHPLC |

