Overcome the Complexities of Analyzing for Sugars by GC-MS
The analysis of sugars spans a wide range of industries and applications and reveals important information in fields such as food safety, medical and diagnostic testing, and environmental testing. Sugars, or carbohydrates, are polar, nonvolatile molecules made up of different arrangements of carbon, hydrogen, and oxygen atoms. The complexities in developing methodologies for sugars lies in the inherent properties of these molecules: their polarity makes running analyses, via LC a challenge, and their low volatility presents difficulties to GC methods. Additionally, sugar molecules do not contain a chromophore, making LC analysis with UV detection a problem. Utilizing a refractive index (RI) detector is a notable alternative for labs who are using HPLC or UHPLC instruments, but RI detectors are known to be less sensitive, less selective, and more sensitive to temperature fluctuations.
The need to develop reliable and efficient methods for analyzing sugars is evident, but what techniques and methodologies can accommodate their unique properties? Derivatization offers an excellent solution in the analysis of sugars: by decreasing polarity, these molecules can be made more LC amenable, and by increasing volatility, they can be made more GC amenable.
To provide guidance in developing optimized methods for analyzing sugars by GC-MS, we evaluated three different derivatization techniques on nine commonly analyzed sugars using the optimized method conditions generated by our Pro EZGC Chromatogram Modeler. Our convenient modeler software is an indispensable tool for laboratories developing and optimizing their own sugar methodologies. The various derivatives were tested according to conditions outlined by our modeler software and evaluated on the basis of efficiency and chromatographic performance. Additional considerations, including the importance of maintenance, are presented to guide method developers in best practices as the various derivatization techniques are explored.
Derivatization Provides a Solution for GC-MS Analysis of Sugars
The derivatization of sugars into their volatile forms provides a means for these compounds to be evaluated by GC-MS analysis. Though there are many different derivatization methods, the most common techniques include silylation and acetylation. Based on this historical body of scientific research in sugar derivation, we chose to evaluate three different techniques: TMS-oximation, TFA-oximation, and alditol acetylation. Trimethylsilyl (TMS) derivatization was identified by Knapp et al. (1979) as a successful technique for derivatizing sugars into their volatile forms1; however, this technique complicates identification and quantification of the subsequent peaks due to the formation of multiple isomers. The number of isomers can be reduced to just two, and separation is improved by adding in an oximation step prior to TMS or TFA (trifluoracetyl) derivatization as demonstrated by Haas et al. (2018)2.
An alternative technique to TMS or TFA oximation, which might be undesirable for some laboratories due to the need to identify and quantify multiple isomers, is alditol acetylation. This technique effectively produces just one derivative peak; however, various sugars may convert into the same derivative peak when carbonyl groups are reduced into hydroxyl groups1. For this reason, it's important to consider the specific sugars being analyzed and their subsequent products after undergoing alditol acetylation. The chemical structures of the resulting derivatives for each of these three techniques are shown in Figure 1 for glucose.
Figure 1: Chemical structures of glucose derivatives from TMS-oximation, TFA-oximation, and alditol acetylation.
Three Alternative Derivatization Techniques: TMS-Oximation, TFA-Oximation, and Alditol Acetylation
To evaluate the efficacy of various derivatization techniques, nine different sugars (rhamnose, mannose, galactose, xylose, fucose, glucose, ribose, fructose, and arabinose) were derivatized by TMS-oximation, TFA-oximation, and alditol acetylation and then separated by GC-MS. The procedure for each of the three techniques is presented in Table I. It should be noted that MBTFA, (N-Methyl-bis(trifluoroacetamide), and BSTFA, (N,O-bis(trimethylsilyl)trifluoroacetamide), are sensitive to moisture, which can present a challenge since sugar molecules are prone to readily absorbing water from the atmosphere. To help prevent any unwanted absorption of water, samples were stored in a dehydrator prior to analysis. Even with the measures taken to avoid water absorption, some challenges were still experienced in initial experiments of TFA-oximation with the MBTFA. To help negate the unwanted effects due to the presence of water, approximately 30-60 mg of 5Å, 8/12, crushed molecular sieve, activated at 300 °C for two hours, and then cooled to room temperature, was added to the MBTFA. Adding the molecular sieve improved derivation using MBTFA; however, peak heights for these samples were slightly reduced.
Table I: Sugar Derivatization Techniques for GC-MS Analysis of Sugars
| Alditol acetylation | TMS- and TFA-Oximation |
|
|
Because TFA- and TFA-oximation are well suited for an automation procedure, a Gerstel Multipurpose Sampler (MPS) was used for TFA-oximation to demonstrate an efficient, time-saving workflow, which is particularly advantageous for labs analyzing numerous samples at one time. See our blog "Derivatization of sugars for GC-MS (Part 4): Automation"3 for additional guidance on performing automation.
Simplify Method Development with Our Pro EZGC Chromatogram Modeler
Determining which derivatization technique best meets your laboratory’s needs is challenging and can be a costly investment of resources. Our Pro EZGC chromatogram modeler contains a comprehensive library of various derivatized sugars to support method developers and researchers in their development of sugar derivation methodologies. This software is also appealing in developing sugar derivatization methods since these derivatization solutions are prone to rapid decomposition. Rather than going through the hassle of performing various trial-and-error runs, which are a costly investment of resources and valuable laboratory time, we were able to take advantage of our modeler to hone in on the optimal method parameters prior to beginning lab work. Conditions were optimized for the Rtx-225 (cat.# 14023) column with a constant flow of helium at 1.4 mL/min, producing good separations and efficient analysis times. The optimized method conditions and model chromatograms generated by our GC modeler are presented in Figure 2.
Figure 2: Optimized run conditions for each group of sugar derivatives generated by the Pro EZGC chromatogram modeler.
|
Ready to take advantage of our EZGC chromatogram modeler to help determine which sugar derivatization method is right for your lab’s needs? Access the modeler at https://ez.restek.com/proezgc Need additional guidance on utilizing the modeler tool? Check out our video series at videos/pro-ezgc-chromatogram-modeler-introduction |
Evaluating the Various Derivatization Techniques on Rtx-225
Each of the three derivatization techniques outlined above were run by GC-MS according to the optimized method conditions generated by our Pro EZGC chromatogram modeler. As expected, both TFA-oximation and TMS-oximation produced two isomer peaks while alditol acetylation produced a single peak for each compound. The results for each of the three derivatization techniques are discussed below. Each methodology has their advantages and disadvantages, and which technique to choose will depend on your lab’s specific needs.
TFA-Oximation
The derivatives from TFA-oximation generated good chromatographic results with the Rtx-225 using the optimized method conditions generated by our Pro EZGC chromatogram modeler. Each sugar derivative was run separately, but an overlay chromatogram is shown in Figure 3 to illustrate peak separations. The rhamnose and arabinose isomers (Peaks 4 and 5) can be expected to have coeluting peaks when run in the same sample (as was predicted from the modeler chromatogram in Figure 2); however, all other isomer peaks were well separated in a total analysis time of under seven minutes. It should be noted that peak height of the sugar derivatives produced using this method was reduced due to retention of the derivatized sugars in adding in molecular sieve, so this derivatization technique is appealing mostly for qualitative purposes. Additional method development is needed for accurate quantification.
Figure 3: Sugar TFA Oximes on Rtx-225
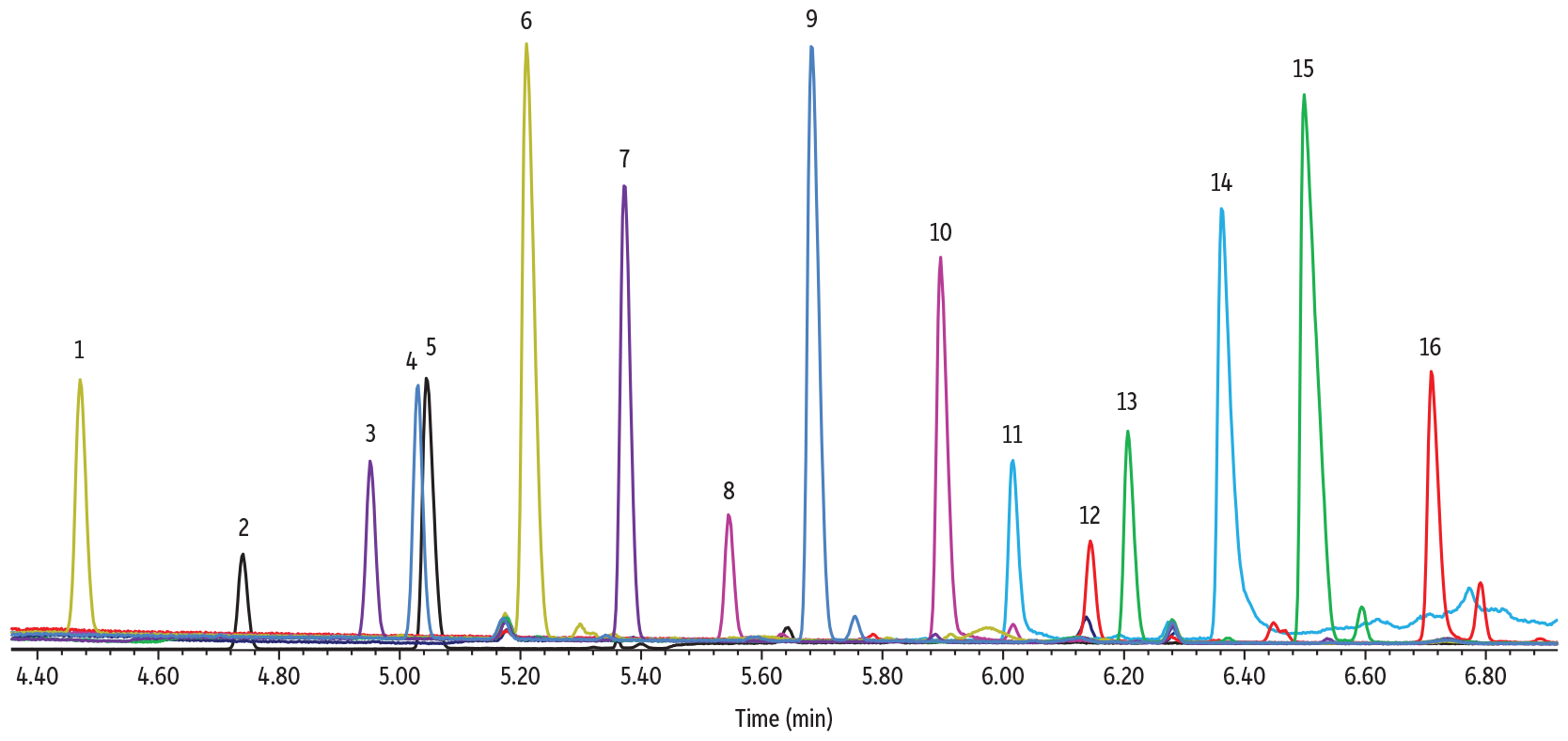
| Column | Rtx-225, 30 m, 0.25 mm ID, 0.25 µm (cat.# 14023) |
|---|---|
| Standard/Sample | Sugar TFA oximes |
| Diluent: | Ethyl acetate |
| Conc.: | 1-5 mg of starting material |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 400:1) |
| Liner: | Topaz 4.0 mm ID Precision Inlet Liner w/ Wool (cat.# 23305) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 80 °C (hold 0.5 min) to 175 °C at 15 °C/min |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Linear Velocity: | 43.739 cm/sec @ 80 °C |
| Dead Time: | 1.21 min @ 40 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | Extractor | ||||||||
| Source Temp.: | 280 °C | ||||||||
| Quad Temp.: | 150 °C | ||||||||
| Electron Energy: | 978 eV | ||||||||
| Solvent Delay Time: | 1.5 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977A MSD | ||||||||
| Notes | Approximately 1 mg of sugar was dissolved in 100 μL of 40 mg/mL ethylhydroxylaminehydrochloride (EtOx) and heated at 70 °C for 30 min. Samples were cooled to room temperature for approximately 5 min. 120 μL of MBTFA (N-Methyl-bis(trifluoroacetamide)) was added to each sample and then heated at 70 °C for 30 min. Samples were diluted 1:100 in ethyl acetate. | ||||||||
TMS-Oximation
TMS-oximation was the most efficient derivatization procedure and the derivatives were more resilient against any effects due to the presence of water than TFA-oximation. Despite a simplified procedure, this methodology required the longest runtime of all three methods (27 minutes) and contained two sets of coeluting peaks that were not easily resolved. The coeluting peaks are shown in Figure 4 below, between peaks 3 and 4, and peaks 13 and 14.
Figure 4: Sugar TMS Oximes on Rtx-225
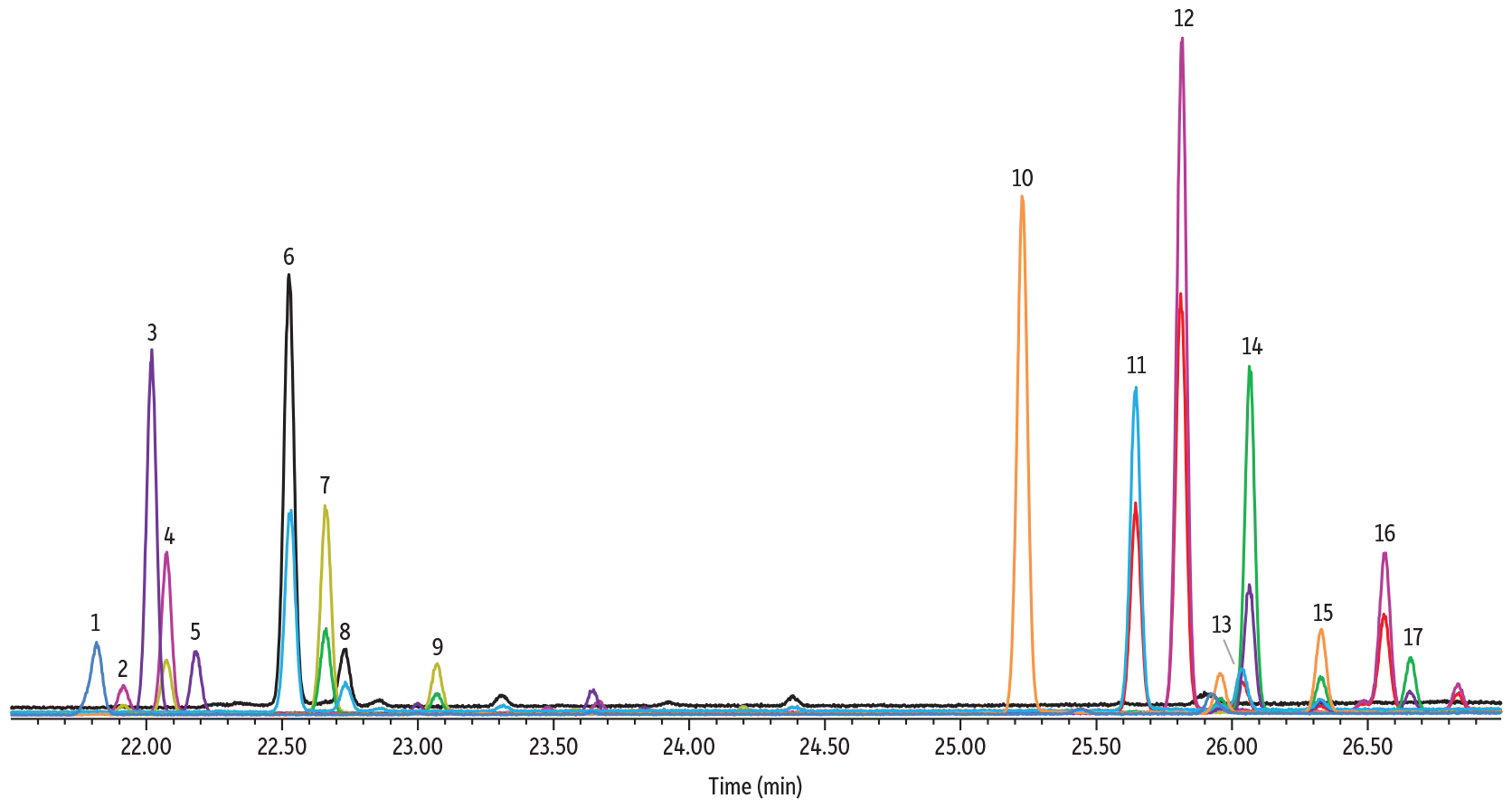
| Column | Rtx-225, 30 m, 0.25 mm ID, 0.25 µm (cat.# 14023) |
|---|---|
| Standard/Sample | Sugar TMS oximes |
| Diluent: | Ethyl acetate |
| Conc.: | 1-5 mg of starting material |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 400:1) |
| Liner: | Topaz 4.0 mm ID Precision Inlet Liner w/ Wool (cat.# 23305) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 0.5 min) to 220 °C at 4.5 °C/min |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Linear Velocity: | 43.4 cm/sec @ 40 °C |
| Dead Time: | 1.14 min @ 40 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | Extractor | ||||||||
| Source Temp.: | 280 °C | ||||||||
| Quad Temp.: | 150 °C | ||||||||
| Electron Energy: | 978 eV | ||||||||
| Solvent Delay Time: | 7 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977A MSD | ||||||||
| Notes | Approximately 1 mg of sugar was dissolved in 100 μL of 40 mg/mL ethylhydroxylaminehydrochloride (EtOx) and heated at 70 °C for 30 min. Samples were cooled to room temperature for approximately 5 min. 120 μL of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) was added to each sample and then heated at 70 °C for 30 min. Samples were diluted 1:100 in ethyl acetate. | ||||||||
Alditol Acetylation
Derivatization by alditol acetylation was a laborious and time-intensive derivatization procedure, but these derivatives showed good chromatographic performance and separations with a total run time under 17 minutes (see Figure 5 below). Generating a single peak, rather than two isomer peaks for each of the various sugars, simplifies identification and quantification. However, depending on the sugars being analyzed, this technique might not be the best option in cases where different sugars will produce the same derivative.
Figure 5: Sugar Alditol Acetates on Rtx-225
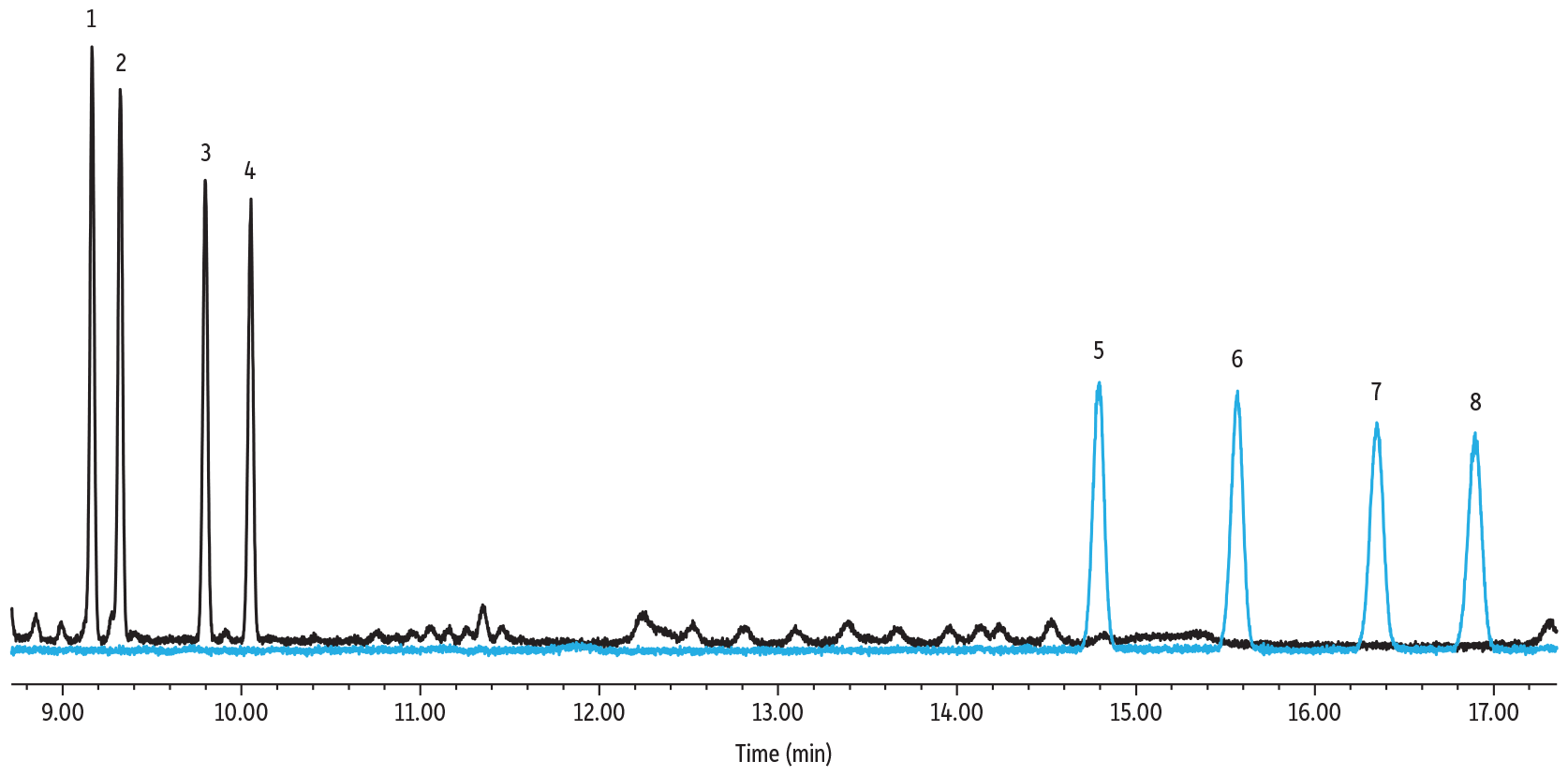
| Column | Rtx-225, 30 m, 0.25 mm ID, 0.25 µm (cat.# 14023) |
|---|---|
| Standard/Sample | Alditol Acetate Mixture-1 (Matreya, LLC, cat.#1124) |
| Alditol Acetate Mixture-2 (Matreya, LLC, cat.#1125) | |
| Diluent: | Chloroform |
| Conc.: | 50,000 µg/mL |
| Injection | |
| Inj. Vol.: | 1 µL split (split ratio 400:1) |
| Liner: | Topaz 4.0 mm ID Precision Inlet Liner w/ Wool (cat.# 23305) |
| Inj. Temp.: | 250 °C |
| Oven | |
| Oven Temp.: | 40 °C (hold 0.5 min) to 220 °C at 30 °C/min (hold 15 min) |
| Carrier Gas | He, constant flow |
| Flow Rate: | 1.4 mL/min |
| Linear Velocity: | 42.906 cm/sec @ 40 °C |
| Dead Time: | 1.21 min @ 40 °C |
| Detector | MS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mode: | Scan | ||||||||
| Scan Program: | |||||||||
| |||||||||
| Transfer Line Temp.: | 280 °C | ||||||||
| Analyzer Type: | Quadrupole | ||||||||
| Source Type: | Extractor | ||||||||
| Source Temp.: | 280 °C | ||||||||
| Quad Temp.: | 150 °C | ||||||||
| Electron Energy: | 978 eV | ||||||||
| Solvent Delay Time: | 1.5 min | ||||||||
| Tune Type: | PFTBA | ||||||||
| Ionization Mode: | EI | ||||||||
| Instrument | Agilent 7890B GC & 5977A MSD | ||||||||
Maintenance Best Practices for Your GC System in Derivatized Sugar Analyses
Performing regular instrument maintenance on your GC system when running sugar derivatives is a vital component in ensuring a successful analysis since derivatized sugars can be especially harsh on you GC system. Troubleshooting issues, such as decreased peak height, widening peaks, and shifting retention times, is time-consuming and can be avoided with regular maintenance to keep your system clean and up and running. In addition to routine maintenance practices, we recommend increased attention to three key areas in your GC system when running sugar derivatives: the syringe, the liner, and column.
Syringe
To ensure a clean syringe, we suggest rinsing both before and after injections with your sample solvent. If rinsing your syringe with a polar solvent, it is also good practice to include a second rinse with a nonpolar solvent, such as hexane or isooctane, to keep the syringe clean from both polar and nonpolar contaminants. Setting up routine rinses for your syringe will prevent the syringe from getting stuck and from carryover contamination in your samples.
Liner
It is crucial to utilize a liner with wool, such as the Topaz 4.0 mm ID Precision Inlet Liner (cat.# 23305), when analyzing for derivatized sugars. A liner with wool is not only a great choice to increase sample volatility, but it also serves as added protection in keeping nonvolatile contaminants from entering the column. Some indications that your liner may be ready to be changed include a visible darkened ring at the top of the liner, decreased peak heights, decreased internal standard recovery, or widened peaks. We found the liner needed to be changed around 40-50 injections during our testing, but if you see any of these signs, it might be time to check your liner. When replacing the liner, it’s also good practice to include replacement of the liner O-ring and the inlet seal.
Column
The derivatizing agents used in these analyses can be especially harsh on GC columns. To help protect your column from the nonvolatile byproducts of the derivatization reaction, we recommend using a guard column, such as our IP Deactivated Guard (cat.# 10049). After numerous injections, it is also good practice to trim the guard column head (approximately 10-20 cm) to help ensure optimal chromatographic performance. A shift in analyte retention times is often an indication that it’s time to trim your guard column (or the column, if a guard is not used).
|
Take advantage of these resources for additional guidance on performing maintenance on your GC system: |
Conclusion
Derivatization provides a solution for laboratories seeking to develop efficient and successful sugar methodologies. Which derivatization method to choose depends on your laboratory’s unique needs, including your compound list, instrument capabilities, and analytical requirements. Our Pro EZGC chromatogram modeler is a powerful tool for helping you determine the most favorable derivatization technique and for optimizing method parameters. Utilizing our convenient modeler for derivatized sugars on the Rtx-225 column eliminates the hassle of performing trial-and-error runs, saves on solvent and consumables use, and helps you to maximize your time in the lab.
References
- D. Knapp, Handbook of analytical derivatization reactions, John Wiley & Sons, 1979.
- M. Haas, S. Lamour, O. Trapp, Development of an advanced derivatization protocol for the unambiguous identification of monosaccharides in Ccomplex mixtures by gas and liquid chromatography, J. Chromatogr. A, 1568, 2018, 160–167. https://doi.org/10.1016/j.chroma.2018.07.015
- E. Pack, Derivatization of sugars for GC-MS (Part 4): Automation, ChromaBLOGraphy, Restek Corporation, 2023. https://www.restek.com/chromablography/derivatization-of-sugars-for-gc-ms-part-4-automation

